THE TERMINAL ARBORISATION OF THE CATS PYRAMIDAL TRACT DETERMINED BY A NEW TECHNIQUE12 [129]
P.D. Wall, W.S. McCulloch, J.Y. Lettvin and W.H. Pitts
##
As neurophysiologists move away from topics of gross organization toward an interest in the finer details of cellular arrangements, it becomes necessary to describe the microscopic distribution of terminal fibers. Gross anatomical methods such as that of Marchi degeneration provide information only about gross connectivity, e.g., that a particular tract loses its myelin in a certain region and therefore probably terminates there. The beautiful Golgi technique tests the extremes of skill and patience of the anatomist. It is particularly difficult to use where the endings of long axons are being examined since the cell body of the axon cannot be seen and the exact origin of an axon cannot be known. However, such workers as Ramón y Cajal, Lorente de Nó, and Sholl have proved the extreme usefulness of the Golgi method. The recent modifications of the Bielschowsky technique by Nauta and Glees have revived interest in the degeneration method of finding endings. The future of this method seems hopeful but there are marked difficulties in technique and interpretation, particularly because of different results obtained by divers workers.
It is also possible to use the conductive properties of nervous tissue to map connections. On the gross level of the Marchi method we have neuronography by strychnine and stimulation, the finding of evoked responses with minimum latency, etc. These tricks are useful for rapid plotting of rough connectivity but also produce their own special problems, some of which are mentioned in another paper (1).
Until recently we have no electrical technique comparable to silver stains because it is difficult to decide what is a relevant signal. The interpretation of slow potentials arising in regions of synapses is most ambiguous — indeed it forms the battleground of opposing theories of synaptic transmission. How such potentials are related to their generators within the signal cord is discussed in another paper (2). Obviously it would be foolhardy to attempt a fine resolution of detail in the presence of such considerable mixing and smearing of signals as occurs under orthodromic stimulation. However, in our work we discovered that it was possible to excite very locally the terminal fibers and record with little decrement the antidromic impulses as digital events in the parent axons.
We describe here results obtained by recording the antidromic volleys generated in terminal arborisations by microelectrode stimulation. By locating each of the large number of stimulation points, we build up a map of the distribution of the endings. The resolution of the method stands between the best anatomical methods and the Marchi technique and is greatly superior to previous physiological methods. It has the advantage of speed and simplicity and can be applied to finding the distribution of endings from a very small number of fibers.
Experimental Technique
The microelectrodes used in these experiments are cylindrical and made of glass-covered platinum wire. The length of the cylindrical thin part was about 5 mm. Two types were used — monopolar electrodes containing 10-micron platinum wire with an overall diameter of about 20 microns, ground to a point at their ends, and bipolar electrodes, containing two separate 10-micron platinum wires, with an overall diameter of 50 microns or less. In the latter type, the tip was ground so that one wire was exactly at the tip and the other emerged on the bevel about 10 microns behind the leading wire. The method of manufacturing these types of electrodes is described elsewhere (1), as is also the method of localizing the electrodes (2). Briefly, the electrodes are pushed into the nerve tissue under the control of a micrometer. The entry is observed under a microscope and the presumed depth of penetration is read off the micrometer. At the maximum depth attained by the electrode, it is cut across with fine scissors and left in place. The cutting is done under the microscope and produces little change in the position of the electrode. At the end of the experiment the tissue is fixed in situ; a slab containing the electrodes is cut out, dehydrated and finally cleared in methyl salicylate. A photograph is then taken of the section and the position and depth of penetration of the electrodes is determined. Only those electrodes whose actual depth in section and presumed depth from the micrometer reading agree to within five per cent are used for plotting results. The electrodes used have a complex impedance in which both the resistance and the reactance vary as the minus 0.95 power of the frequency. When the frequency is above 5 kc, the electrode resistance is lower than the tissue resistance. The stimulator used generated constant-current pulses about 0.2 msec. long.
The current flowing from the tip of the electrode was adjustable from 0.1 to 50 µa but was usually kept at about 0.5 to 5 µa. Since even light barbiturate anesthesia increases the threshold of terminal afferent fibers, the experiments were carried out wherever possible in spinal or decerebrate cats. Evoked motor movement was controlled either by curare or by ventral root section. The segments of the spinal cord to be examined were exposed by the usual laminectomy and covered with oil. The temperature of the animal and of the bathing oil was controlled throughout the experiment.
Experimental Results
Localization of large, sensory nerve fiber endings and motor horn cells in the grey matter of the lumbar cord.
In this set of experiments the accuracy of the localization of the stimulus was first tested by stimulating motor horn cells. In the right-hand diagram of Figure 1 the results are shown. Recording electrodes were placed on the cut ventral root of the first sacral segment. Stimulating microelectrodes were lowered into the spinal cord. The black dots show the points from which direct responses on the ventral root were evoked by direct stimulation of the motor horn cells. The dotted line represents the extreme of penetration of the electrodes in this experiment. Of course, responses occurred in the ventral root when many other parts of the cord were stimulated but these were delayed by more than a millisecond after the stimulus, implying synaptically evoked activity of the motor horn cells. It is clear from the distribution of responsive points, first, that the resolution is quite good since no responses were evoked by stimulation in the white matter immediately adjacent to the ventral horn and, second, that dendrites were not being stimulated. The distribution shown is, as histologists agree, that of the motor cell bodies and axons. We know from other experiments that the point of stimulation on these cells has a threshold which is modified by the arrival of afferent nerve volleys. This modification of excitability differs if anodal, rather than cathodal, stimulating pulses are sent through the monopolar type of microelectrode. It seems likely, therefore, that different parts of the cell are being stimulated, depending on the sign of the stimulating current. Impulses were generated in the terminals of afferent fibers, at the same time as in the motor horn cells, and passed antidromically out the dorsal roots. Presently, a large number of studies on the endings of sensory fibers from various peripheral nerves in the grey matter of the cord have been done. Here, however, we wish to show only one example.
In a number of cats the lumbar enlargement was exposed and all lumbar and sacral ventral roots on one side were cut. Next, the nerves to the gastrocnemius were dissected out, cut, and placed on recording electrodes. A series of microelectrodes were lowered into the cord and, at 100-micron intervals during the descent of each one into the cord, a stimulus was given. If a response on the peripheral nerve of gastrocnemius occurred within 1.4 milliseconds, it was taken as an indication that intraspinal fibers belonging to the large (group 1) sensory fibers from the gastrocnemius nerve were present close to the tip of the stimulating microelectrode. Since all the motor roots had been cut, there was no chance of confusion between the motor and sensory components of the nerve. We see in the left hand diagram of Figure 1 the deep penetration of these fibers into the ventral horn. This distribution of sensory-fiber endings contrasted markedly with the slower conducting sensory fibers which terminated in a band across the ventral part of the dorsal column or the slowest conducting fibers which terminated in the substantia gelatenosa. The threshold of the endings of the group I fibers in the ventral horn was surprisingly low, being about the same as that of the motor horn cells.
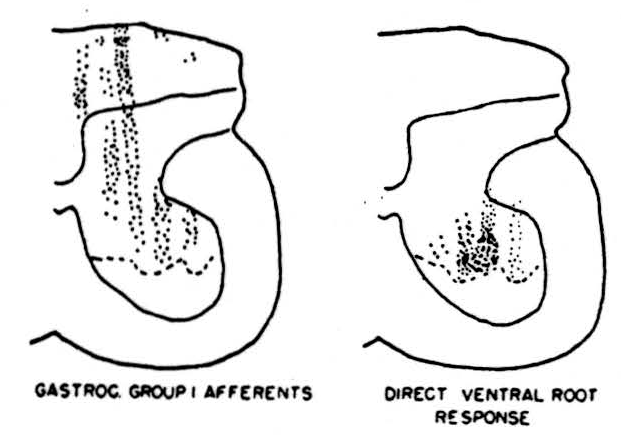
Figure 1. Left: distribution of group 1 fibers from the nerve to gastrocnemius in the grey matter of the first sacral segment of the cord; determined by multiple microelectrode placements for stimulation and recordings taken from the peripheral nerve after sectioning of motor roots. Right: distribution of points from which direct stimulation of motor horn cells was obtained; dotted lines represent the deepest penetration limit of the microelectrodes so that no data were collected from the area ventral to this line.
Localization of the endings of the pyramidal tract in the spinal cord.
Recordings were taken from the pyramidal tract 2 mm. behind the pontomedullary junction. The most useful type of recording bipolar electrode was an insulated nichrome wire in a hypodermic needle tube (27 gauge). The end was shaped to a point by setting it in Stycast plastic resin and grinding it down to the required shape after polymerisation. The electrode was lowered into position either by Horsley Clarke coordinates or more simply by removing some of the posterior midline cerebellum and inserting the electrode 1 mm. lateral to the visible midline of the floor of the fourth ventricle. The position of the electrode was checked at the beginning of the experiment by stimulation of the motor cortex before decerebration and at the end of the experiment by histological examination. Next, the cervical enlargement was exposed and microelectrodes were lowered one after the other into the cord. A stimulus was delivered through the microelectrode at each stage of the penetration until the more ventral parts of the cord were reached, after which the electrode was cut off and left in place for later localization. Figure 2 shows the distribution of the electrode positions in the grey and white matter which produced a response in the pyramidal-tract recording electrode in the medulla. This is one of seven experiments in cats, all of which showed essentially similar results. In this experiment nine electrodes were used; the three most medial ones failed to evoke any antidromic volleys in the pyramidal tract, while the six more lateral ones evoked responses in the area indicated in the figure. In this and in the other experiments very minor responses were found from a slightly more extensive area although never extending into the ventral horn. The responses recorded in the marked area were definite even at the edges and increased in size rapidly as the electrode was moved toward the center of the marked area. The edge of the area was remarkably sharp, both with bipolar electrodes and with monopolar electrodes, provided the current flow was controlled. The simplest way of checking the extent of current spread was to place the stimulating electrode just to one side of the midline in the dorsal columns and check on relevant dorsal roots for spread of the stimulus across the midline to the contralateral dorsal column. In this way, it was possible to maintain a small area of stimulus even with monopolar electrodes. The monopolar electrodes had two advantages, first, greater ease of manufacture than the bipolars and, second, the interesting finding that they were far more efficient in stimulating white matter, presumably because the bipolars failed to stimulate many myelinated fibers because of the absence of nearby nodes.
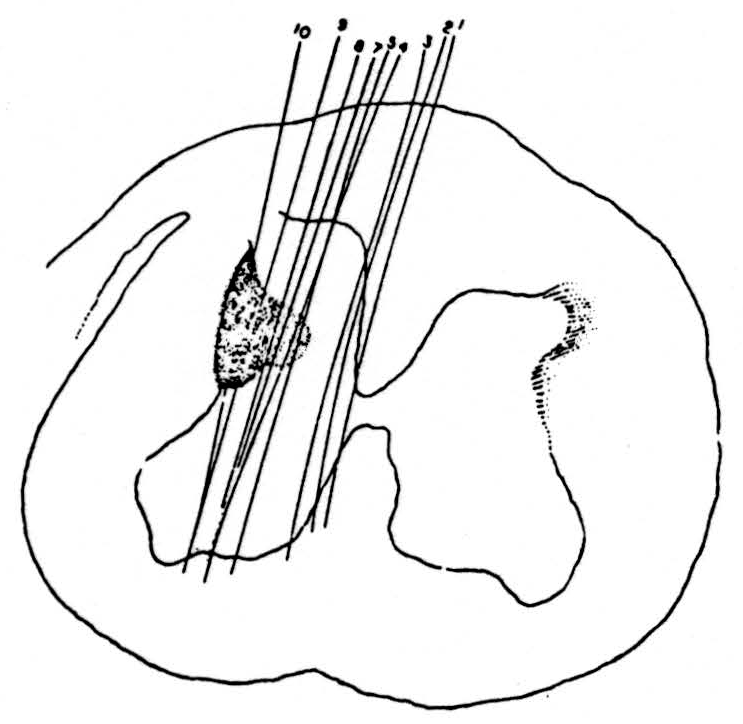
Figure 2. Distribution of points in a lower cervical segment of cat spinal cord from which microelectrodes could elicit antidromically-traveling impulses recorded in the medullary pyramid. This shows the extremely limited extent of the penetration of the terminal arborisation of the pyramidal tract into the spinal cord grey matter. Conduction speed of the antidromic impulses about 65 m/sec.
Three facts should be noted. First, the area in which the pyramidal tract appears to end does not extend into the ventral or extensively into the dorsal horns. Second, it was not possible to demonstrate the presence of a direct pyramidal tract in the lower end of the cervical region of the cat although this may have been caused by the localizing nature of the pick-up electrode in the medullary pyramids. Last, there were no clear signs of projections into the pyramidal tract of ascending direct sensory fibers. This does not mean that no other waves were recorded from the electrode in the pyramidal tract but it was clear from their size, timing and maximum, determined by moving the electrode, that these were impulses traveling in the adjacent medial lemniscus and other white matter. No signs of pyramidal projection into the lumbar enlargement could be found in the cat. In one rhesus monkey the terminations of the pyramidal tract in the cervical enlargement were again found to be similar to that in the cat with no signs of projection onto the ventral horn cells. The distribution of endings in the monkey extended slightly more ventrally and medially into the intermediate grey than in the cat.
Discussion
Our technique involves the penetration of a series of microelectrodes into the area to be searched. We have placed up to fifteen monopolar electrodes in a half cross-section of the spinal cord without being able to detect any change in the reflex pattern involving that segment of the spinal cord. It is evident, therefore, that the damage produced by the electrodes does not severely upset the intraspinal organization. As a further check against damage, we have frequently inserted the final electrode of an experiment into the middle row inserted during the previous few hours. The results obtained from this interpolated electrode are consistent with the pattern established by its neighbors so that there are no signs of progressive damage. Rarely, a change was seen during an experiment and on examining the cord later we have found a small petechial hemorrhage surrounding one of the electrodes. However, this is so rare that it is clear that the collision of the electrodes with blood vessels does not usually result in bleeding.
With monopolar electrodes, and to a lesser extent with bipolar electrodes, it is obvious that if the current strength is high enough, distant parts of the nervous system may be stimulated. As we mentioned above, the current strength was controlled to obtain the optimum resolution. If there existed within the spinal cord gross inhomogeneities of electrical resistance, the use of a fixed stimulating current might give spurious localization of responding points. We have examined this by mapping the current distribution within the cord from a single point source. While it is true that there is a difference in resistance between the white and grey matter, this inhomogeneity does not significantly distort the localization of terminals which we describe in this paper.
On looking at the very short distance of penetration of corticospinal fibers into the dorsolateral grey the question may be asked: “How sure are we of stimulating the collaterals all the way to their terminals?” Not only have we the results of tracing the dorsal root afferents all the way to the ventral horn, but we have mapped pari passu with the pyramidal fibers the distribution of the medial longitudinal fasciculus. The latter gives rise to very long collaterals ascending from the ventral columns to the very edge of the dorsal columns; the method of recording in the brainstem was exactly the same as that used for the pyramids.
Thus, for the cat, we have definitely confirmed the impression of others that the intraspinal fibers of the pyramidal tract end in the deeper part of the dorsal horn and extend slightly into the intermediate region. No signs of invasion of the ventral horn could be seen. This agrees with the anatomical findings of Rasdolsky (3) and the physiological findings of Lloyd (4). We were unable to find any signs of ascending sensory fibers within the pyramidal tract as reported by Brodal and Walberg (5), and by Brodal and Kaada (6). In all experiments signs of ascending sensory volleys were seen but on more careful localization they were found to be running in tracts dorsal to the medullary pyramids.
Unfortunately, we cannot make as definite a statement on the endings in the rhesus monkey. Hoff and Hoff (7) reported anatomical studies showing a few scattered endings of the pyramidal tract in the ventral horn. Bernhard, Bohm, and Petersen (8) have recently reported monosynaptic facilitation of the motor neurons in the monkey. It is evident from their writings that they had some difficulty in evoking this monosynaptic effect and it seems usually to have been necessary to send long bursts of impulses down the pyramidal tract from the cortex before the effect could be seen. In the one experiment we have been able to carry out in the monkey, it is true that the area containing endings extended more ventrally and extensively into the intermediate region than in any of the cats examined. However, the area did not include the ventral horn segments in which Hoff and Hoff (7) show endings and could at best have connected with some long meandering dorsal dendrites of some motor horn cells. Thus, while it is clear that the pyramidal tract in the monkey is far more developed than in the cat, especially in its invasion of lumbar segments, we have not been able to confirm that its intraspinal organization is radically different from that of the cat, although this point requires further investigation
Summary
- A new technique is described which uses antidromic impulses generated in terminal arborisations by stimulating microelectrodes. The position of the stimulating microelectrode is determined for those points of stimulus which evoked a response recorded in a tract projecting into the stimulated area. The resolution of the technique is shown to be greatly superior to previous physiological methods which attempted to localize the ending of projection fibers.
- The technique is used to show the endings of the group I afferent fibers from the gastrocnemius muscle in the grey matter of the cord.
- The ending of the pyramidal tract in the cat is shown to be limited to a small region in the ventral part of the dorsal horn. Preliminary results on the monkey are also discussed.
Footnotes
References
Lettvin, J.Y., McCulloch, W.S., Pitts, W.H. and Wall, P.D.: Some Effects of Strychnine. Epilepsia, 1955, in press.
Howland, B., Lettvin, J.Y., McCulloch, W.S., Pitts, W.H., and Wall, P.D.: Reflex Inhibition by Dorsal Root Interaction. J. Neurophysiol., 1955, 18, 1.
Rasdolsky, J.: Uber die Endigung der Extraspinalen Bewegungsysteme. Z. gest. Neurol. Psychiat., 1923, 86, 361.
Lloyd, D.P.C.: The Spinal Mechanism of the Pyramidal System in Cats. J. Neurophysiol., 1941, 4, 525.
Brodal, A and Walberg, F.: Ascending Fibres in the Pyramidal Tract of Cats. Arch. Neurol. Psychiat., (Chicago), 1952, 68, 755.
Brodal, A and Kaada, B.R.: Exteroceptive and Proprioceptive Ascending Impulses in the Pyramidal Tract of Cat. J. Neurophysiol., 1954, 16, 567.
Hoff, E.C. and Hoff, H.E.: Spinal Terminations of Projection Fibres from the Motor Cortex of Primates. Brain, 1934, 57, 454.
Bernhard, C.G., Bohm, E., and Petersen, I. Organization of the Corticospinal System in Monkeys, Acta physiol, scand., 1952, 23, 79.
For further research:
Wordcloud: Area, Bipolar, Cat, Cells, Cord, Current, Cut, Distribution, Dorsal, Electrodes, Endings, Evoked, Experiment, Fibers, Figure, Finding, Grey, Hoff, Horn, Impulses, Localization, Lowered, Matter, Method, Microelectrodes, Monkey, Monopolar, Motor, Nerve, Penetration, Points, Position, Pyramidal, Recording, Region, Responses, Results, Root, Segment, Sensory, Signs, Spinal, Stimulating, Stimulus, Technique, Terminal, Tract, Used, Ventral, Wire
Keywords: Methods, Technique, Organization, Tract, Cat, Method, Degeneration, Fibers, Arrangements, Axons
Google Books: http://asclinks.live/jy2b
Google Scholar: http://asclinks.live/e631
Jstor: http://asclinks.live/0wk2