A CEREBELLO-BULBO-RETICULAR PATHWAY FOR SUPPRESSION[^*][^+] [88]
R.S. Snider, W.S. McCulloch and H.W. Magoun
##
Recent studies on the cerebrum by Dusser de Barenne and McCulloch (1) and the medulla oblongata by Magoun (2) indicate that these suprasegmental regions can act to suppress certain functions of the nervous system. More recent studies indicate that at least one of the cerebral suppressor areas (i.e., 4s) is connected by a direct pathway to the bulbar suppressor area (3). In view of the fact that Sherrington was able to suppress the extensor tone of decerebrate animals by electrical stimulation of the anterior cerebellar lobe (4), and since it has been known for many years that a pathway extends from the nuclei fastigii of the cerebellum to the medulla oblongata—the fastigio-bulbar pathway—it was possible, theoretically at least, that there exists a functional relationship between certain parts of the cerebellum and the suppressor area of the medulla oblongata. The present paper is concerned with experimental data indicating that such a relationship does exist.
Methods
Most of the animals used in these experiments were normal cats placed under a surgical level of Dial (Ciba) or Nembutal anesthesia. In approximately one-third of the experiments, the animals were decerebrated at a red nucleus level, vinethene anesthesia being applied during the surgical procedures. Electrical activity was amplified through a Grass 6-channel amplifier and was visualized on a cathode-ray tube. The amplitude of the cortically induced (cerebral) movement or the patellar reflex response was recorded by means of an ink-writing kymograph; the reflex was evoked by a solenoid operating recurrently on a timing circuit. Electrical stimulation of the various parts of the nervous system was carried out by means of a Goodwin stimulator. Silver electrodes of the bipolar type were used for surface stimulation. Recording electrodes whose maximum diameter was I mm. consisted of two nichrome wires which were insulated except at the tips. These electrodes were oriented with the Horsley-Clarke stereotaxic instrument. Lesions were made electrolytically, when desired, by passing 3 ma of current through “unipolar” electrodes for 1 minute. Microscopic examination of frozen sections of the explored areas of the nervous system stained according to a Weil myelin sheath method permitted identification of the sites either stimulated or destroyed.
Results
Suppression of cortically induced motor responses
Stimulation of cortex of anterior cerebellar lobe. That electrical activation of the cerebellar cortex of the anterior lobe will suppress cortically induced (cerebral) movement in the cat is amply shown by Figure 1A, IB and 1C.
In Figure 1A, electrical stimulation of the pericruciate area of the cerebrum with 4.2 volts at 60 pulses per sec., produced abduction of forepaw every 20 seconds. Simultaneous stimulation of the culmen with 20 volts produced no noticeable suppression of the cortically induced movement, but 30 volts produced a slight effect, and 35 volts abolished the response entirely.
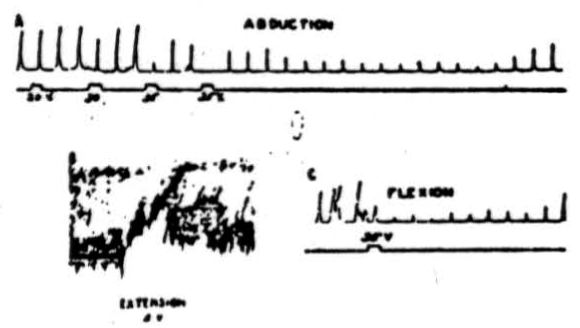
Figure 1. A: Electrical stimulation (every 20 sec.) of cerebral pericruciate area of cat under deep Nembutal anesthesia, with 4.2 volts at 60 pulses/sec., to produce abduction of forepaw—simultaneous stimulation of culmen with 20, 30 and 35 volts at 300/sec. Note suppression of cortically induced movement and long post-stimulatory effects. B: Electrical stimulation (every 20 sec.) of cat's pericruciate area under Nembutal anesthesia, with 2.0 volts at 60/sec., to produce extension at wrist—simultaneous stimulation of posterior part of anterior cerebellar lobe with 5 volts at 300/sec. Note suppression of cortically induced movement. C: This record was obtained under experimental conditions similar to those used in A , except that in this case flexor rather than abductor movement was elicited. Note suppression of cortically induced movement.
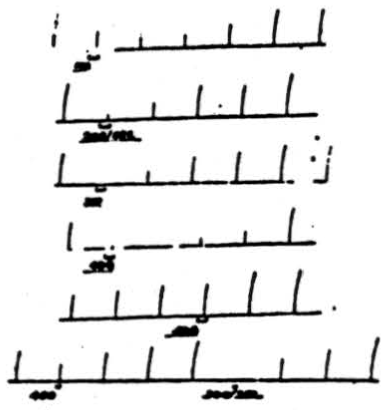
Figure 2. Effect of changing frequency of cerebellar stimulation upon cortically induced movement. Flexion of right rear leg was induced by applying 4 volts, 50/sec., 10 sigma to pericruciate cortex once per minute for 1 sec. Simultaneous stimulation of anterior cerebellar lobe at (a) 40 volts, 100/sec. for 8 sec.; (b) 40 volts, 200/sec. for 8 sec.; (c) 40 volts, 300 /sec. for 8 sec.; (d) 20 volts, 400/sec. for 8 sec.; (e) 20 volts, 500/sec. for 8 sec.; (f) 20 volts, 40Ö /sec. for 4 sec.; (g) 20 volts, 300/sec. for 4 sec. Note that for a given voltage applied for a given duration, frequency of 300/sec. is most effective one.
In the post-stimulatory period the response came back almost to normal, fell again, and it was many seconds afterward before the responses returned to normal amplitude. This animal was under deep Nembutal anesthesia, which undoubtedly accounts in part, at least, for the high voltages necessary to activate the cerebellum. In Figure 1B is shown the effect of anterior cerebellar lobe stimulation on extensor muscles. In this case 2.0 volts at 60 pulses per sec. were applied to the pericruciate area of the cerebral cortex to produce extension of the wrist. As can be seen from the recorded responses, simultaneous excitation of the posterior part of the anterior cerebellar lobe with 5 volts, 300 pulses per sec., produced profound diminution in height of the responses as well as a decrease in tone. The cerebellar effect continued for several seconds after withdrawal of the stimulus. The experimental record shown in Figure 1C was obtained under conditions similar to those already listed for Figure 1A except that in this case cerebral stimulation produced a flexion. Simultaneous cerebellar stimulation depresses the cerebral induced movement, and it remains temporarily depressed, as was the case in Figure 1A. Thus Figure 1A, IB and 1C shows that anterior cerebellar lobe stimulation can suppress abductor, extensor, and flexor movements, and although the record is not shown, suppression of adductor movements has also been observed.
Throughout these experiments high frequencies of stimulation have been applied to the cerebellar cortex because they proved more effective, as is shown in Figure 2. Cortically induced movement (flexion of right rear leg) was induced by applying 4 volts at 50 per sec. and 10 sigma for 1 second once each minute. If 40 volts at 100 per sec. are applied to the anterior cerebellar lobe for 8 seconds, a slight suppression is produced. However, if the frequency of electrical stimulation is increased to 200 per sec., a greater suppression is produced. If, as is shown in the third record, the frequency of stimulation is increased to 300 per sec. (all other coordinates remaining unchanged), complete suppression of the response results. Now, if the voltage of cerebellar stimulation is reduced to 20 and applied for 8 seconds at 400 per sec., suppression is almost complete. However, if the frequency is increased to 500 per sec., there is very little effect. If, at the same time, cerebellar stimulation at 20 volts is applied for 4 seconds at 400 per sec., there is slight suppression which becomes complete when the frequency is reduced to 300 per sec. Thus, in both cases, i.e., 40 volts for 8 seconds and 20 volts for 4 seconds, frequencies of 300 pulses per sec. prove the most effective for suppressing cortically induced movement. In some animals stimuli at 200 per sec. were equally effective. A similar statement can be made about suppression of reflex activity.
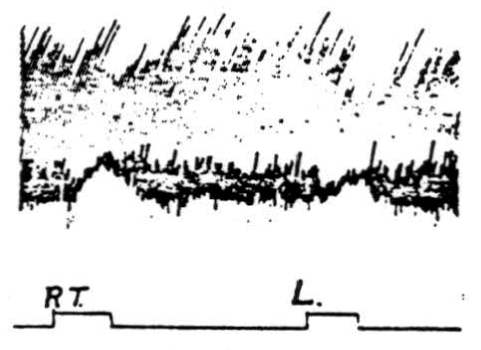
Figure 3. Inhibition of cortically induced movement by simultaneous stimulation of paramedian lobules. Electrical stimulation of pericruciate area of cerebrum of cat under Nembutal anesthesia with 4-5 volts, 30 /sec., .2 sigma to produce extension of left wrist. Simultaneous cerebellar stimulation of right (Rt.) and of left (L.) paramedian lobule with 20 volts, 300/sec., 0.2 sigma produced suppression of movement.
Stimulation of paramedian lobules and surrounding folia. As shown in Figure 3, suppression of cortically induced movement may be obtained by simultaneous stimulation of the paramedian lobule of the cerebellum. The pericruciate area (cerebral) stimulus was 4.5 volts (2 sigma) at 30 per sec., and the cerebellar stimulus was 20 volts (2 sigma) at 300 per sec. The stimulus was applied to the left paramedian lobule (ipsilateral to the extensor
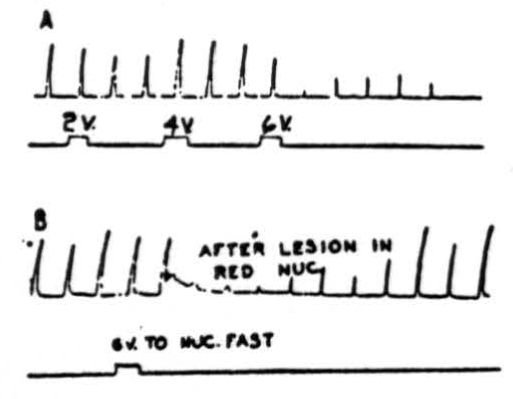
Figure 4. A: Effects of electrical stimulation of nucleus fastigii on cortically (cerebral) induced movement. Abduction of right hind leg of cat under Nembutal anesthesia was produced by applying 4.5 volts, 30/sec., 5 sigma every 20 sec. Suppression of movement was produced by stimulating right nucleus fastigii with 6 volts at 300 pulses/sec.; 2 volts and 4 volts were not effective. Rarely, activation of nucleus fastigii contralateral to movement produced suppression. Note that suppression (after 6 volts) continued for 3.5 minutes after removal of stimulus. B: Same preparation as A except that red nuclei have been destroyed bilaterally, does not interrupt pathway.
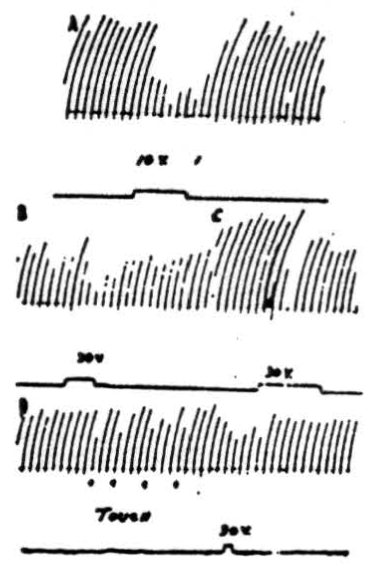
Figure 5. A: Effects on patellar reflex of stimulation of anterior cerebellar lobe. Patellar reflex of cat under Dial anesthesia was elicited every 5 sec. by means of a solenoid and recurrent timer. Simultaneous stimulation of culmen at junction with lobulus centralis with 10 volts, 100/sec., 2 sigma produced reduction of reflex response. B: Effects on patellar reflex of stimulation of ipsilateral paramedian lobule. Patellar reflex of cat under Dial anesthesia was elicited every 5 sec. Ipsilateral paramedian lobule was stimulated with 30 volts at 100/sec. C: As B except paramedian lobule contralateral to movement was stimulated. In lightly anesthetized animals voltages as low as 10 volts could be used to produce suppression. D: At 4 points marked “TOUCH” inhibition of knee jerk was produced by lightly touching a cotton swab to pial surface of ipsilateral paramedian lobule. Note that response was decreased by mechanically stimulating cerebellar cortex. Electrical stimulation of same area also caused a diminution of knee jerk.
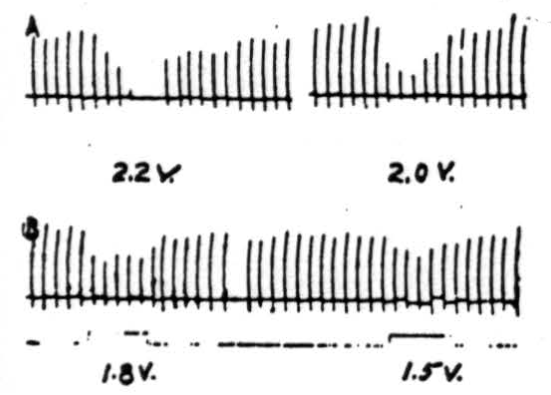
Figure 6. Effects of electrical stimulation of nucleus fastigii on reflexly induced movements. Patellar reflex of cat under Nembutal anesthesia was elicited every 5 sec. Electrical stimulus of 2.2 volts (300/sec., 0.2 sigma) applied to ipsilateral nucleus fastigii stopped response, while stimuli of 2.0 volts, 1.8 volts, or 1.6 volts depressed its amplitude. Similar stimulation of nuclei interpositus and dentatus did not suppress patellar reflex.
movement of the wrist). It was slightly less effective when applied contralaterally—thus indicating that the paramedian suppressor area exerts its influence bilaterally.
Stimulation of nucleus fastigii. A question then arose as to which of the cerebellar nuclei relays the impulses from the cerebellar cortex to lower centers. That the nucleus fastigii is the chief relay nucleus is shown by Figure 4. In the experiment illustrated in Figure 4A, abduction of the right hindleg was produced by electrically stimulating the left pericruciate area of the cerebral cortex with 4.5 volts at 30 per sec. every 20 seconds. Stimulation of the ipsilateral (occasionally contralateral as well) nucleus fastigii with 6 volts, 300 per sec., produced depression of the cortically induced movement. As can be seen in this experiment 2 and 4 volt stimulations had little effect on the responses. Note that suppression continued for many seconds (in this case, 3.5 minutes) after termination of the cerebellar stimulus. That the red nucleus was not a necessary part of the suppressor pathway is shown in Figure 4B, in which suppression of cerebral induced movement occurred following stimulation (6 volts, 300 per second) of the ipsilateral nucleus fastigii after the red nuclei had been destroyed bilaterally.
Suppression of reflex activity
Stimulation of the cortex of the anterior cerebellar lobe. In Figure 5A is shown the result of a typical experiment in which the knee jerk was mechanically elicited every 5 seconds by means of a solenoid and recurrent timer. Simultaneous stimulation of the anterior border of the culmen, at the junction with lobulus centralis, with 10 volts (100 per sec., 2 sigma) produced obvious reduction of the reflex response. Stimulation with higher voltages suppressed the responses completely. The most pronounced effects were obtained from electrical stimulation of the ipsilateral anterior lobe although occasionally weak contralateral effects could be detected.
Stimulation of cortex of paramedian lobules and surrounding folia. That inhibition of the patellar reflex can be induced by electrical stimulation of paramedian lobules and surrounding folia is dearly shown in Figure 5B, 5C and 5D.
In the experimental record shown in Figure 5B 30 volts at 100 per sec. were applied to the paramedian lobule, ipsilateral to the knee jerk, for the period of 4 knee jerks, or slightly more than 20 seconds. As can be seen from the record, responses during the period of stimulation were definitely decreased in amplitude. Stimulation with higher voltages stopped the responses altogether. In lightly anesthetized animals complete suppression was obtained with stimuli as low as 10 volts. That stimulation of the contralateral paramedian lobule near the junction with the pyramis also suppresses the patellar reflex is shown in Figure 5C. Note that suppression does not persist during the entire period of stimulation at this voltage (30 volts), which is the same as that applied to the other side. Although it was possible to stop the knee jerk temporarily by applying higher voltage to the cerebellum, contralateral stimulation at a given voltage was never so effective as was ipsilateral stimulation.
Figure 5D shows an interesting record which demonstrates that suppression can be produced not only by electrical stimulation of the cerebellar cortex but by mechanical means as well. At the 4 points marked “touch” a cotton swab was gently applied to the ipsilateral paramedian lobule at the site electrically stimulated in Figure 5B. Each time the pial surface was touched, there was a decreased reflex response.
Stimulation of nucleus fastigii. As shown by the records in Figure 6, the patellar reflex was elicited every 5 seconds. Simultaneous electrical stimulation with 2.2 volts applied to the ipsilateral nucleus fastigii completely suppressed the responses, while 2.0 volts reduced them considerably (in magnitude), and 1.8 and 1.5 volts reduced them slightly. All stimuli to the nucleus were given at 300 per sec. with 0.5 sigma falling phase. Rarely small inhibitory effects could be obtained from the contralateral nucleus fastigii. No effects were observed from the stimulation of the nuclei interpositus or dentatus of either side.
Electrical evidence
The results of exciting various parts of the cerebellum and recording the altered electrical activity of the bulbar reticular formation by means of a cathode-ray oscilloscope (bipolar pick-up electrodes), are shown in Figure 7. In Figure 7A the cortex of the posterior part of the anterior lobe was stimulated with a single shock at 3 volts and the record illustrates the resulting activity of the ipsilateral bulbar reticular formation. Following the shock artefact (sharp downward deflection) there are three major waves following each other in rapid succession. The peak of the first occurs about 1 msec. after the stimulus and the peaks of each of the other two follow at about 0. 6 msec. intervals. Following the third wave there is sometimes a period about as long as that covered by the three waves during which the spontaneous activity is slightly diminished.
In the experiment illustrated in Figure 7B, the cerebellar cortex was stimulated at the junction of the pyramis and tuber vermis. Except for a small diphasic response of about 1 msec. latency, no altered electrical activity was recorded.
In Figure 7C the ipsilateral nucleus fastigii was stimulated with 2 volts and the record demonstrates the altered activity of the bulbar reticular formation. Note that unlike the record shown in Figure 7A, there is only one large wave which peaks at about 0.3 msec. A slight second and third wave may occasionally appear on the downward slope of the wave, but never were the three waves sharply separated as in Figure 7A. Obviously, the latency of discharge recorded from the bulb is shorter in stimulation of the fastigial nucleus than when the cerebellar cortex is activated. Thus, it is obvious that the cerebellar cortex and the nucleus fastigii can activate the bulbar suppressor area.
The photomicrograph shown in Figure 8 is included to illustrate further evidence concerning the suppressor pathway from the cerebellum. Electrical stimulation in the cerebellum along electrode tracts A or B or in the bulbar reticular formation at the bottom of electrode tracts A or B produced suppression of knee jerk or cortically induced (cerebral) movement. Then an electrolytic lesion was made at C, as shown in the photograph. Subsequent
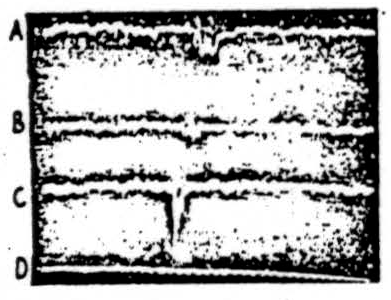
Figure 7. Electrical activity recorded from ipsilateral bulbar reticular formation while stimulating various parts of cerebellum. A: Electrical stimulation, with 3 volts, of posterior part of anterior lobe of cat under Dial anesthesia. Bipolar pick-up electrodes were in bulbar reticular formation and cathode-ray recording was photographed directly from tube. Note shock artefact and multiple response. B: As A except that cerebellar stimulus was applied at junction of paramedian lobule and tuber vermis. Note shock artefact and small response. C: As A except cerebellar stimulus was applied directly to ipsilateral nucleus fastigii. Note proximity of response to shock artefact. D: Time scale—1000/sec.
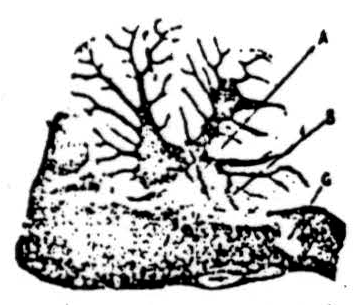
Figure 8. Electrical stimulation in cerebellum and in bulbar reticular formation along electrode tracts at A or B produced suppression of reflexly or cortically induced movement. Then an electrolytic lesion was made at C and subsequent stimulation along electrode tracts A or B failed to produce suppression, thus indicating that descending pathway for suppression was interrupted by lesion at C.
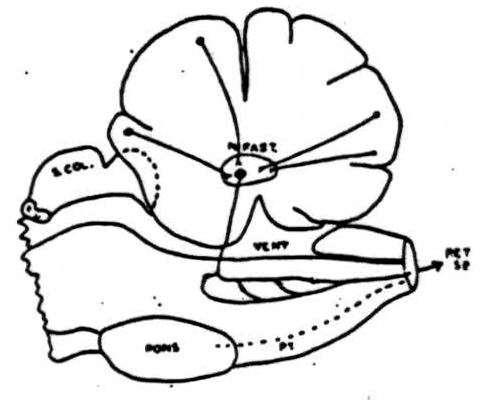
Figure 9. Summary of pathways involved in cerebellar suppression of reflexly induced and cortically (cerebral) induced movements. Cells in cortex of anterior cerebellar lobe and paramedian lobules (plus pyramis and surrounding folia) send axons to nucleus fastigii where they synapse with cells whose axons pass to reticular formation of medulla where another synapse takes place and impulses pass down into cord via reticulo-spinal tracts. This system is chiefly, but not entirely, an ipsilateral one.
stimulation in the cerebellum and bulbar reticular formation along electrode tracts A and B failed to produce suppression. It is clear, therefore, that the lesion at C destroyed an intermediate structure in the pathway from the cerebellum to the spinal cord. Upon microscopic analysis this structure proved to be the caudal part of bulbo-reticular formation and reticulospinal tracts descending from it.
An outline drawing summarizing our conception of the pathways involved in cerebellar suppression of reflex and cortically induced (cerebral) activity is shown in Figure 9. Cells in the cerebellar cortex of the anterior lobe and the paramedian lobule discharge into the nucleus fastigii. This, in turn, projects to the bulbar reticular formation where synapses are made with cells whose axons pass down into the spinal cord as reticulo-spinal tracts. This system is chiefly an ipsilateral one and its interruption anywhere between the cerebellum and spinal cord prevents cerebellar suppression of reflexes or cortically induced movement.
Discussion
Numerous workers have repeated Sherrington's original observation (4) that electrical stimulation of the anterior cerebellar lobe inhibits the extensor tone of decerebrate rigidity and in the present studies it was easy to make similar observations. However, so far as we are aware, this is the first work which shows that cerebellar mechanisms are intimately related to the bulbar reticular formation and act through it to cause inhibition at a spinal level. From our studies it is clear that the pathway from the cortex of the anterior lobe passes through the nucleus fastigii, where it probably synapses; then, as the fastigio-bulbar pathway, it passes into the bulbar suppressor area, where another synapse takes place with cells forming reticulo-spinal tracts, which have been shown by Rhines and Magoun (5) to carry fibers of suppressor function to the spinal cord.
The anterior lobe is not the only cerebellar area which upon stimulation yields suppression because, as Bremer (6) has pointed out, similar effects can also be obtained from the pyramis. In our studies a second inhibitory area was found to be bilaterally represented in the paramedian lobules and pyramis. Electrical stimulation of this area produced suppression of cortically induced and reflexly induced movements identical to that produced by anterior lobe stimulation. The pathway from the paramedian lobules, like that from the anterior lobe, passes through the nuclei fastigii to the bulbar reticular formation.
At this point it is necessary to emphasize that stimulation of the two suppressor areas of the cerebellum suppresses not only the extensor tone of decerebrate rigidity but such cortically induced movements as extension, abduction, and flexion, as well. These data, when interpreted in the light of the influence of the cerebellum on the cerebrum (7) and in the light of our own work on cerebellar facilitation (8), give a versatility to cerebellar function not hitherto suspected. It is obvious that the conclusions which we have drawn as a result of the present experiments are strongly supported by the work of other investigators in this field. As was pointed out earlier in this paper, Sherrington as far back as 1898 (4) demonstrated inhibition of extensor tone of decerebrate rigidity. Bremer (6) was able to obtain inhibition not only from the anterior lobe but also from the pyramis. Allen (9) in a foresighted review indicated the possibility of a cerebellar relationship with the bulbar reticular formation and pointed out that inhibition might result from such a functional relationship. Moruzzi (10) extended the work further when he observed that cerebral-induced movement could be inhibited by cerebellar stimulation.
Electrical stimulation of the cerebellar cortex introduces an interesting series of phenomena in the bulbar reticular formation (see Fig. 7). Instead of a single wave, a single stimulus evokes a series of three or four waves. When this is contrasted with the electrical phenomena resulting from the stimulation of the nucleus fastigii, it can be seen that only one wave of short latency results. Therefore, the multiple waves arise in the cortical circuits or nerve cells. An analysis of these repetitive responses of cortical activities may lead to an understanding of cerebellar function in terms of its fast intrinsic activity. In this connection, it is well to remember that stimulation is most effective with frequencies as high as its spontaneous activity.
When the suppressor areas of the cerebellum are mapped with the tactile areas described by Snider and Stowell, they are nearly coextensive—closely resembling motor areas described by Hampson, Harrison and Woolsey (11) and are homologous with the facilitatory areas as observed in the monkey (8). The presence of motor, sensory, inhibitory, and facilitatory areas confined to these parts of the cerebellum reminds one of another region of the central nervous system where this peculiar combination of functional activities is located—namely, the pericruciate area of the cerebrum of carnivores. Yet, when the deficits of animals with lesions in the pericruciate areas are contrasted with those of animals with lesions in appropriate areas of the cerebellum, there is a striking dissimilarity in behavior. Obviously, more work is necessary before this interesting series of observations can be fitted into a smoothly functioning unit.
Summary
- Studies on the suppression of reflexly induced and cortically induced (cerebral) movements by electrical activation of the anterior cerebellar lobe and paramedian lobules plus nearby folia are reported.
- Frequencies of 200 to 300 pulses per sec.—usually 300 per sec.—were found to be most effective for cerebellar stimulation.
- The pathway concerned with suppression from the cerebellum is: cerebellar cortex to nucleus fastigii to bulbar reticular formation, thence to the spinal cord via the reticulo-spinal tracts.
- Evidence is presented which shows that the suppressor areas, the facilitatory areas, and the tactile areas of the cerebellum are practically coextensive.
Footnotes
1 Reprinted from J. Neurophysiol., 1949, 12:325-334. (This work was presented in its entirety before the American Physiological Society at its annual spring meeting in 1947.)
+ Aided by a grant from the Office of Naval Research.
References
Dusser de Barenne, J. G. and McCulloch, W. S. Suppression of motor response upon stimulation of area 4s of cerebral cortex. Amer. J. Physiol., 1939, 126: 482.
Magoun, H. W. Bulbar inhibition and facilitation of motor activity. Science, 1944, 100: 549-550.
McCulloch, W. S., Graf. C., and Magoun, H. W. A cortico-bulbo-reticular pathway from area 4s. J. Neurophysiol., 1946, 9: 127-132.
Sherrington, C. S. Decerebrate rigidity and reflex coordination of movements. J. Physiol., 1898, 22: 319-332.
Rhines, R, and Magoun, H. W. Brain stem facilitation of cortical motor response. J. Neurophysiol., 1946, 9: 219-229.
Bremer, F. Contributions è l'étude de la physiologie du cervelet. La fonction inhibitrice du paleo-cérébellum. Arch. int. Physiol., 1922, 19: 189-226.
Walker, A. E. An oscillographic study of cerebello-cerebral relationships. J. Neurophysiol., 1938, 1: 16-23.
Snider, R. S. and Magoun, H. W. Facilitation produced by cerebellar stimulation. Fed. Proc., 1948, 7: 117-118.
Allen, W. F. Formatio reticularis and reticulospinal tracts, their visceral functions, and possible relationships to tonicity and clonic contractions. *J. Wash. Acad. Sci., 1932, 22: 490-495.
Moruzzi, G. Sui rapporti fra cervelletto c corteccia cerebrale. III. Meccanismi e localizzazioni delle azioni inibitrici e dinamogeni del cervelletto. Arch. Fisiol., 1941, 41: 183-206.
Hampson, J. L., Harrison, C. R., and Woolsey, C. N. Somatotopic localization in the cerebellum. Fed. Proc., 1946, 5: 41.
Snider, R. S. and Stowell, A. Receiving areas of the tactile, auditory, and visual systems in the cerebellum. J. Neurophysiol., 1944, 7: 331-357.

Warren McCulloch and Jerome Lettvin Picnic at the McCulloch Farm Old Lyme, Connecticut

(l. to r.) Walter Pitts, Jeannie Davidson, Warren McCulloch, Tim Davidson, 1949

Walter Pitts, 1949

Warren McCulloch and Jerome Wiesner, 1953

Jack Cowan and Leo Verbeek, 1959 M.I.T. Lab
For further research:
Wordcloud: Activity, Anesthesia, Anterior, Applied, Area, Bulbar, Cat, Cerebellar, Cerebellum, Cerebral, Cortex, Cortically, Effects, Electrical, Electrodes, Fastigii, Figure, Formation, Frequency, Induced, Inhibition, Ipsilateral, Lobe, Lobule, Movement, Note, Nucleus, Paramedian, Patellar, Pathway, Per, Pericruciate, Produced, Record, Reflex, Response, Reticular, Sec, Seconds, Shown, Sigma, Simultaneous, Stimulation, Stimulus, Suppression, Suppressor, Tracts, Voltage, Volts, Wave
Keywords: Lobe, Pathway, Areas, Cerebellum, Studies, Area, Life, Oblongata, Paper, Animals
Google Books: http://asclinks.live/llg6
Google Scholar: http://asclinks.live/msvd
Jstor: http://asclinks.live/ewfz