FUNCTIONAL ACTIVITY AND pH OF THE CEREBRAL CORTEX12 [19]
J.G. Dusser de Barenne, W.S. McCulloch and L.F. Nims
Seven Figures
In a previous paper by Brody and Dusser de Barenne (1) it was established that the motor response to electrical stimulation of the cortex of the cat was markedly enhanced during hyperventilation of the animal. Following electrical stimulation of the cortex Dusser-de Barenne and McCulloch(2, 3) found a few years ago a temporary inactivation of the cortex, extinction, which under special types of stimulation, lasts for several minutes. It was considered highly probable that among the factors underlying these changes in excitability were changes in the pH of the cortex. In a recent preliminary paper(4) a method has been briefly described, showing that it is possible to measure the pH of the cerebral cortex. In this paper we wish to report more fully on the relations of the pH of the cortex to its activity and excitability under various experimental conditions including electrical stimulation.
Methods
The method used gives a continuous record of the pH of the cortex, its D.C. potentials, the respiration of the animal (natural or artificial), time and signals. This is done by photographing upon moving paper a screen (fig. 1, S) upon which are focused four light beams: two from Leeds and Northrup galvanometers (no. 2420), one for the pH, the other for the D.C. potential, the third from a mirror on a Marey tambour moving with the respiration, and the fourth recording time and signals.
At the times indicated on this record simultaneous electrocorticograms² have been obtained with amplifiers, a Westinghouse four-element oscillograph and its moving-paper camera. In detail the method was as follows (fig. 1) :
The pH is measured by the EMF developed between a glass electrode and an adjacent wick-saline electrode on the cortex.
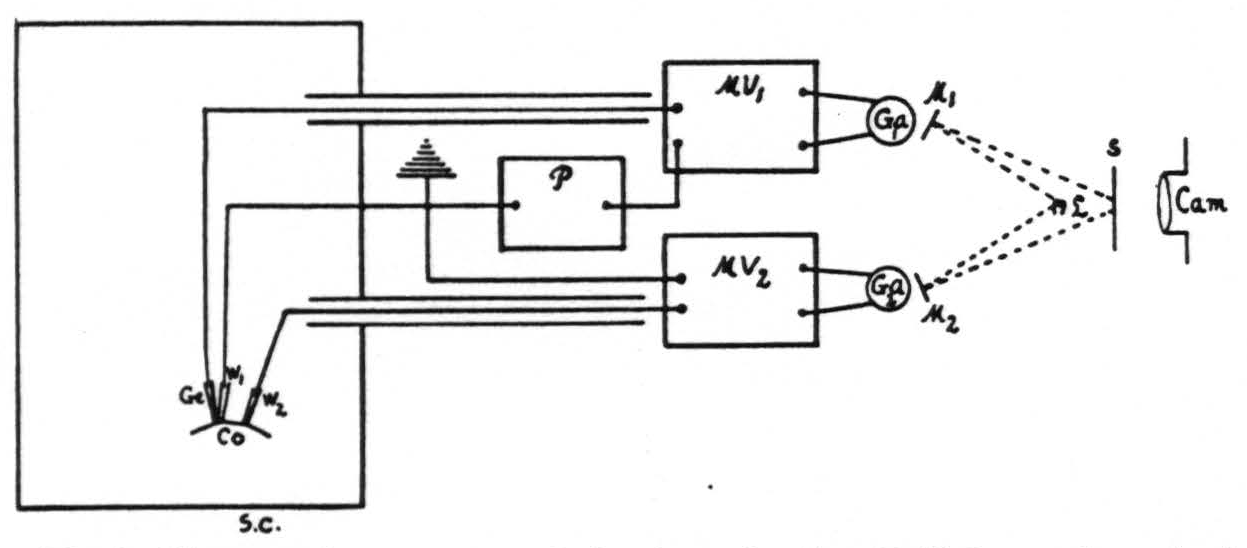
Figure 1. Diagram of apparatus and circuit used. Sc, shielded cage for animal. Co, cortex. Ge, glass electrode. W₁ and W₂, wick electrodes. P, potentiometer. MV₁ and MV₂, vacuum tube microvoltmeters. Ga₁ and Ga₂, galvanometers. M₁ and M₂, mirrors of Ga₁ and Ga₂. L, light source. S, ground glass screen. Cam., camera for moving paper with its F/1.25 anastigmatic lens.
The measuring circuit used consists of a potentiometer (P), a vacuum tube microvoltmeter (MV₁) and a galvanometer (Ga₁). The glass electrode is patterned after that of MacInnes and Dole (5) with a concave active area (Corning glass 015) less than 0.5 mm. in diameter. This electrode, supported by a spring so as to exert a small constant pressure on the cortex, effectively seals from contact with the air the minute volume of cerebro-spinal fluid between the electrode and the cortex.
The glass electrode is filled with a silver acetate-acetic acid buffer solution, into which projects a silver wire (fig. 2). This combination was found to give a stable electrode, which in the range of biological pH does not reverse polarity.
In ordinary pH determinations it is customary to use a saturated KCl salt bridge. Obviously this cannot be done on
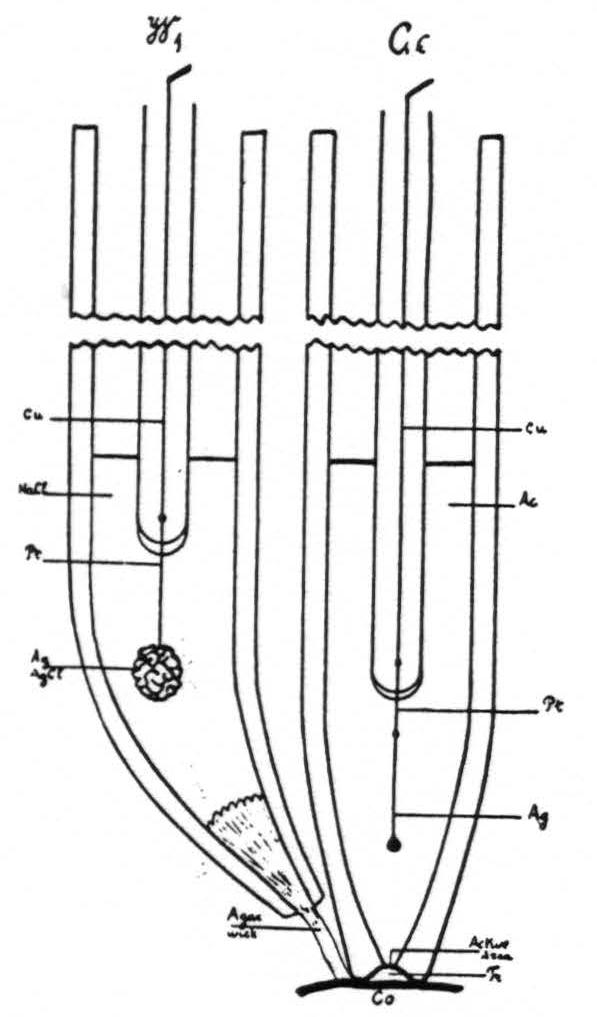
Figure 2. Ge, diagram of glass electrode. Cu, copper wire. Pt, platinum wire. Ag, Ag wire. Ac, silver acetate-acetic acid buffer. Tr, minute quantity of transudate underneath glass electrode. Co, cortex. W₁ wick electrode. NaCl, normal saline solution.
living tissue. Therefore, a physiologically normal NaCl solution was used as a bridge, into which dips a silver-silver chloride electrode (fig. 2, W₁), contact with the cortex being established through an agar wick. This system was carefully checked against phosphate and acetate buffers of known pH and found to be reproducible and to give the required differences in EMF (±0.01 pH).
The EMF of a phosphate buffer (pH = 6.83) was measured before and after each experiment and these determinations were found to agree within 1 millivolt; this shows that during the course of an experiment, even extending over many hours, the voltages were accurate within this limit. The phosphate buffer was assigned its pH value from measurements with a hydrogen-ion electrode in accordance with the pH scale described by Hitchcock and Taylor (6).
The measuring circuit is truly potentiometric, in which the potentiometer (fig. 1, P) at the start of each experiment was set so as to balance the voltage developed between the electrodes. The microvoltmeter (fig. 1, MV₁) of the type described by Burr, Lane and Nims (7) with a grid leak of 100 megohms makes it possible to measure the voltage accurately and does not pass enough current to polarize the glass electrode even when the potentiometer is several millivolts off balance. Thus the circuit is in balance when the galvanometer spot is in the center of the screen (fig. 1, S) and any deflection, indicating variation in voltage, is recorded continuously without further adjustment of the potentiometer. The deflections are found to be a linear function of the voltage differences.
Since the cortex exhibits D.C. potential gradients, it was essential to obtain a record of these gradients also. This was done with a separate microvoltmeter (MV₂), galvanometer (Ga₂) and two wick electrodes (W₁, W₂) as described above, one of which was also used for the pH measurement (fig. 1). These D.C. potential gradients were found small enough, even after electrical stimulation of the cortex, to be negligible in estimating its pH.
Whenever desired, a separate record of the electrical activity of the cortex was obtained, always through condensers, in order to insulate the animal from extraneous D.C. currents. From one to four channels of capacity-coupled amplifiers with various arrangements of pick-up electrodes were used in conjunction with a Westinghouse four-element oscillograph and a separate moving-paper camera. Corresponding times and signals were indicated on both records from a common signal circuit.
In those experiments in which electrical stimulation of the cortex was performed, it was always done with 60 cycle A.C. through condensers, disconnected from the animal except during the stimulation. Sixty cycle A.C. was used, 1) because its symmetrical wave form reduces polarization to a minimum, 2) because its long wave form results in a prolonged, marked after-discharge in the cortex even with small voltages.
The animal was always placed on a board, insulated by paraffin blocks from the shielded, grounded cage in which it was placed. The animal was grounded at one point only, i.e., through the wick electrode common to the pH and D.C. circuits. The glass electrode was always placed adjacent to this common wick electrode on the surface of the cortex; the position of the other electrodes was dictated by the experiment in question.
It is essential to ground, to shield and to insulate not only the animal, but also all other circuits involved.
All experiments reported in this paper were performed upon monkeys (Macaca mulatta). Usually the animals were fully anesthetized with Dia1,³ sometimes with the addition of curare. In a few animals the operative procedures were performed under ether; in these instances the animals during the actual experiment, were curarized.
Results
Before presenting the results of these experiments we wish to state that in measuring the pH of the transudate on the surface of the cortex, one obtains a close approximation of the pH of the tissue subjacent to the glass electrode. A simple experiment proves this point. Thermocoagulation at 80°C. for 5 seconds of a small area of the cortex renders this killed area acid (e.g., pH = 6.6), while the pH of the immediately adjacent, normal cortex remains unchanged (e.g., pH = 7.3). An area so treated goes through two stages of vascularity. For the first 10 to 15 minutes after the thermocoagulation the area is blanched and presents a maximal vasoconstriction, subsequently the area becomes markedly hyperaemic and oedematous. Although the blood supplied to the cortex is indirectly a factor in determining the pH of the transudate, the above observation shows that the measured pH reflects primarily the acidity of the tissue, rather than changes in its vascularity or changes in the pH of the blood supplied to the cortex. This is not surprising, when one considers that the lymphatic drainage from the cortex is to the surface.
The results here presented are the following:
Hyperventilation of the animal, by removing CO₂ from the blood, induces an increase of the pH of the cortex. This is evident in figure 3.
Hypoventilation of the curarized animal, resulting in accumulation of CO₂ in the blood, leads to a decrease of the pH of the cortex. See also figure 3. In fact it is possible to adjust the artificial respiration of the animal to any desired pH of the cortex compatible with life.
These changes in pH are reflected also in typical changes in the electrocorticogram.
A fresh specimen under light Dial narcosis and breathing spontaneously maintains a pH fluctuating between 7.2 and 7.4. When such an animal is artificially ventilated, with or without curare, so that the pH of the cortex is maintained at 7.3, increase of ventilation is associated with an increase of the pH of the cortex and an increase of its ’spontaneous’ electrical activity.
With small increases of pH this augmentation in the electrocorticogram is chiefly in amplitude (fig. 4), with larger increases of pH and increase in frequency with a diminution in amplitude. A decrease in ventilation resulting in a lower pH of the cortex is associated with a decrease in both amplitude and frequency of the cortical action potentials (fig. 5). In extreme cases of ‘acidity’ of the cortex the electrocorticogram is practically obliterated. The quantitative relations involved are now under investigation.
Intravenous injection of a few cubic centimeters of a sodium bicarbonate solution increases the pH of the cortex, injection of a few cubic centimeters of dilute hydrochloric acid decreases its pH. Again the electrocorticogram follows the pH of the cortex as indicated in paragraph 3: high pH inducing increased electrical activity, low pH inducing decreased activity (fig. 6).
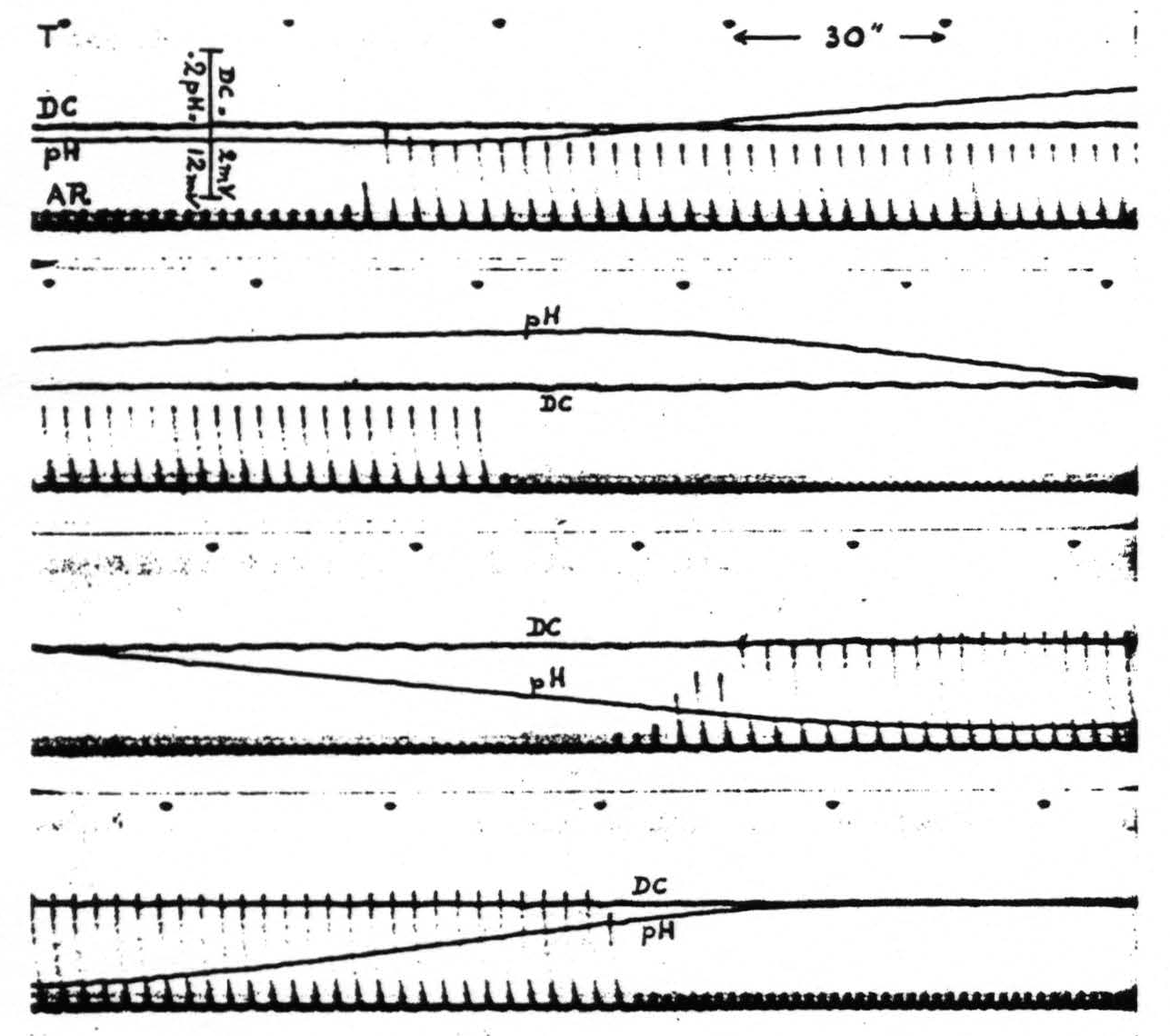
Figure 3. Monkey. Light Dial narcosis. Tracheal cannula. Curare. The four strips are continuous. T is time in half minutes. AR is artificial respiration. DC is potential between two wick electrodes about 2 mm. apart on cerebral cortex. pH is variation of potential between glass and one of the wick electrodes, also about 2 mm. apart on cortex; upward increase, downward decrease of pH.
Note that changes in artificial respiration are followed by shifts in pH without appreciable shift in D.C. potential, although the D.C. voltage sensitivity is six times as great.
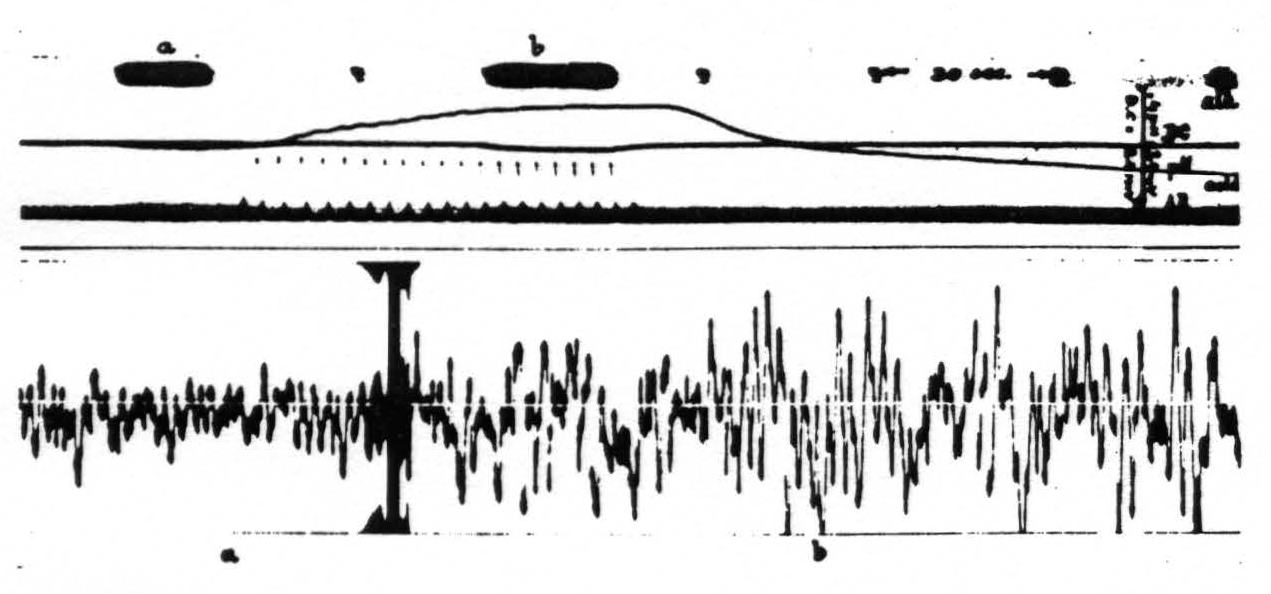
Figure 4. Monkey. Dial narcosis. Curare. Artificial respiration. D.C. potential and pH of cortex and artificial respiration (AR) recorded in upper part of picture. Below electrical activity of cortex: a, during normal artificial respiration; b, during hyperventilation, resulting in alkalinity of cortex. During subsequent hypoventilation pH of cortex shifts toward acid side.
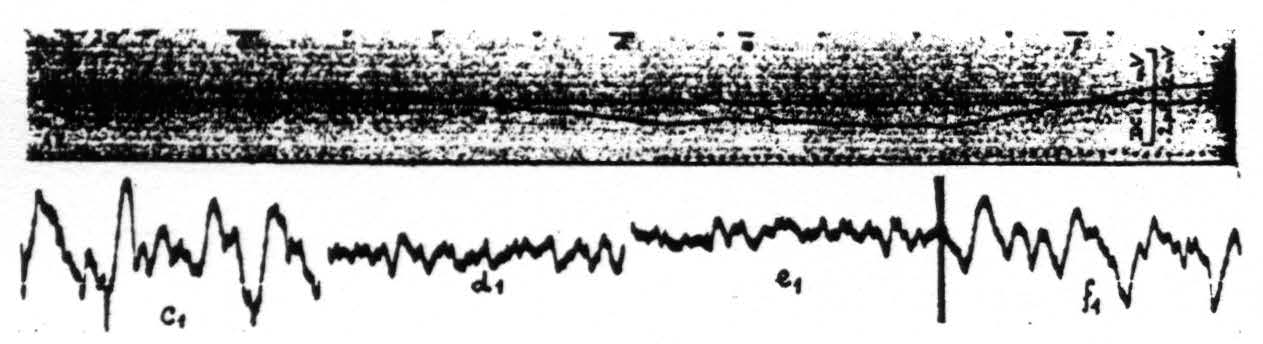
Figure 5. Monkey. Dial. Curare. Upper record shows the changes in pH of the cortex under different ventilation. Downward shift of pH record is one toward acid side. Lower records electrocorticograins during these changes in pH. c₁ taken at period marked c in upper record, d₁ during d, e₁ during e and f₁ during f.
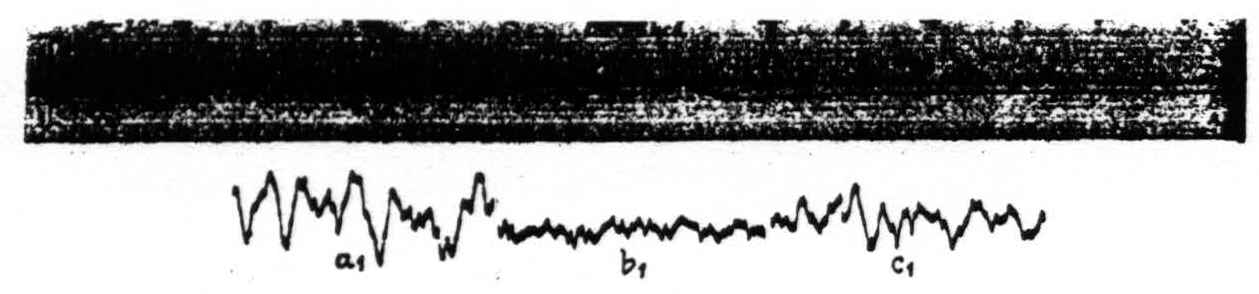
Figure 6. Monkey. Dial. Curare. This figure demonstrates the influence of an intravenous injection of dilute acid upon the pH of the cortex (upper record). The three lower records show the electrical activity of a cortical focus at various pH levels. Record a₁ taken during the period marked a in the upper record, b₁ during b and c₁ during c in the upper record.
In fact, the level of electrical activity seems to be determined largely by the pH of the cortex, irrespective of the manner in which the changes of the pH are brought about.
Intravenous injection of a few cubic centimeters of physiological saline solution, which does not change the pH of the cortex, does not alter its electrical activity.
Stimulation of the ‘motor’ cortex with 60 cycle A.C. for 5 seconds (bipolar) at an appropriate voltage results in an after-discharge in the electrocorticogram. This electrical after-discharge appears whether or not the connections of the cortex with the lower levels of the CNS have been severed by under-cutting.
Using this after-discharge as evidence for cortical excitation, the voltage required for a minimum of after-discharge is a measure of the excitability of the cortex proper. Increased pH of the cortex, however induced, results in a marked increase of its excitability; with an increase of pH from 7.3 to 7.5 the voltage threshold was found lower by more than 25% of its initial value.
During decreased pH of the cortex, its voltage threshold was found markedly increased. The changes in threshold are more marked with lower values of pH; the quantitative aspects of the relationships of pH to threshold of the cortex are being studied.
These findings provide experimental verification of the statement of Brody and Dusser de Barenne (1), that under hyperventilation the excitability of the cortex is enhanced.
When the shift in pH of the cortex is produced systemically, e.g., by a change in the ventilation, a given electrical after-discharge in the cortex results in a smaller peripheral motor after-discharge when the pH of the cortex is low than when it is high. Inasmuch as the cortical excitation is constant here, the difference in the peripheral response can be accounted for only by concurrent similar changes in pH and excitability at lower levels of the CNS. It is important to note that these changes in excitability in response to alterations of pH hold for neural as well as for electrical excitation. Such changes in excitability to neural excitation are sufficient to account for the observed changes in the electrocorticogram induced by changes in pH.
Older observations in the literature suggest and Lehmann’s recent quantitative studies (8) demonstrate that similar changes occur in peripheral nerve.
In the experiments of Brody and Dusser de Barenne (1) a temporary depression of excitability followed the cessation of hyperventilation and lasted as long as 4 to 5 minutes. In a monkey under similar experimental conditions (Dial anesthesia, not curarized, artificial respiration) on returning from hyperventilation to the initial ventilation, a pronounced and prolonged acid swing with decrease of the electrocorticogram was observed. As stated above, this shift is accompanied by a decrease of cortical excitability, thus substantiating the original observation of 1932.
Electrical stimulation of a focus of the motor cortex such as to produce electrical after-discharge results in a marked decrease of the pH of the cortex in the immediate neighborhood of the focus. This local ‘acidity’ develops during the after-discharge and outlasts it considerably. It is sufficiently large to account for the subsequent reduction or quiescence in the electrocorticogram. In fact the electrocorticogram gradually resumes its original characteristics (fig. 7) only as the pH of the cortex returns to its prestimulatory level.
These findings have been established in the non-curarized as well as in the curarized animal; the muscular after-discharge, with its production and discharge into the circulation of metabolites, is, therefore, not an essential factor; the local ‘acidity’ following efficient electrical stimulation of the cortex must be due to the activity, the ‘firing’ of the thousands of nerve cells under the stimulating electrodes.
Not only the cortical focus stimulated becomes ‘acid,’ but also, although to a lesser extent, distant foci so functionally related to the first focus that in them also electrical after-discharge appears. Thus, stimulation of a focus of arm area 4 (precentral, A₄) results not only in a decrease of the pH in this area, but also in a definite fall of pH in the postcentral arm area as far occipital as A₇. No change in pH was found in cortical areas in which no after-discharge appeared.
The time elapsing between the beginning of the hyper- or hypoventilation and that of the shift in pH of the cortex, which can appropriately be called the latency of these changes in pH, measures in the narcotized (Dial) monkey usually between 8 and 12 seconds. In the non-narcotized animal the latency of the pH shifts was, as expected, much shorter, between 2.5 and 5 seconds. The shifts were also larger for a
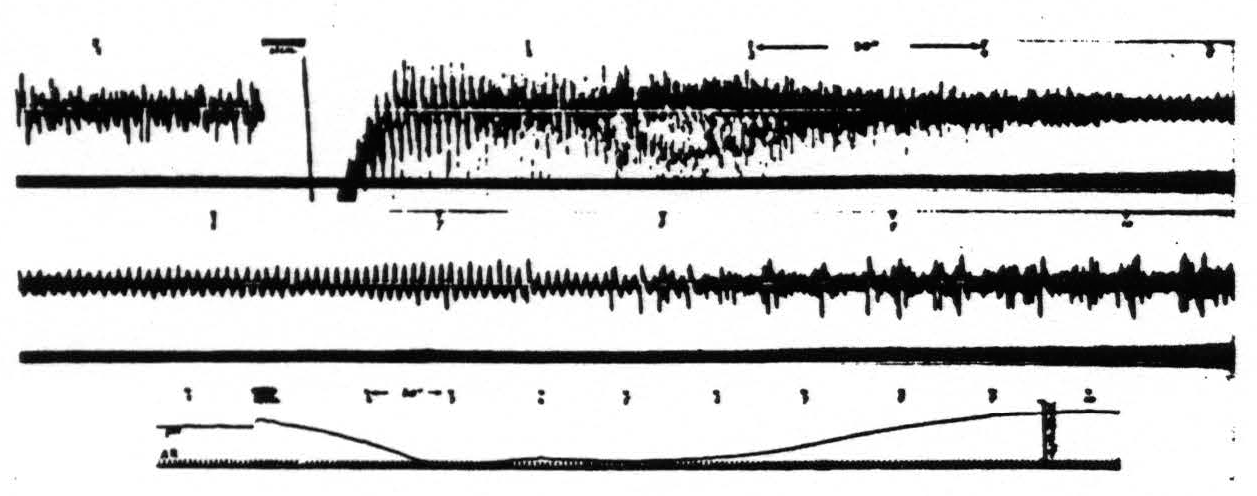
Figure 7. Monkey. Dial narcosis. Artificial respiration (AR in record 3). Records 1 and 2 continuous action potential records from an arm 4 focus before and after stimulation (5″, 60 ∼, VD = 8000). Record 3 gives corresponding pH changes. Poststimulatory hyperactivity manifests itself in electrical after-discharge and results in development of acidity at focus. Acidity once established reduces electrical activity. Numbers indicate corresponding times.
given variation of the experimental conditions than in the narcotized animal. In this latency are comprised several factors, 1) of an instrumental nature (galvanometer, equilibration of the fluid confined underneath the glass electrode) and 2) physiological. If one considers that we measure the pH on the surface of the cortex, in all probability of its normal transudate, while most of the activity reflected in the pH of this transudate occurs in the deeper layers, this rather long latency is not surprising. Most of this latency is accounted for by the slowness of diffusion through the cortex and into and within the droplet of cerebro-spinal fluid confined underneath the glass electrode. It should be emphasized that while one does measure the actual pH of the cortex in any steady state, the observed pH during a change lags and probably never reproduces indicates as great a change as that which occurs at the site of disturbance.
In conclusion it seems advisable to point out that in experiments of this nature one must realize that stimulation of those cortical areas which control respiration and autonomic functions (i.e., especially area 6) produces alterations of pH and excitability which complicated the picture. For this very reason the experiments reported are those in which area 6 was not involved.
Summary
- A method is described for recording the pH of the cerebral cortex with simultaneous electrocorticogram.
- Hyperventilation or intravenous injection of sodium bicarbonate in the curarized animal under artificial respiration (constant in the second instance) results in a marked ‘alkaline’ shift of the cortex.
- Hypoventilation or intravenous injection of a dilute acid in the curarized animal under artificial respiration (constant in the second instance) results in a marked ‘acid’ shift of the cortex.
- Increased pH (‘alkalinity’) of the cortex results in an increase of its electrical activity, decreased pH (‘acidity’) in a decrease of its electrical activity.
- During increased pH of the cortex its excitability is increased, during decreased pH its excitability is decreased. The same is true of lower levels of the NS, and for neural as well as for electrical excitation.
- These changes in excitability, following changes in pH, are sufficient to account for the observed changes in electrical activity of the cortex.
- After-discharge in the cerebral cortex results in a decreased pH at the site of the after-discharge, whether this is induced electrically by direct electrical stimulation of the focus or neurally by a disturbance propagated from a distance.
Footnotes
Literature Cited
Brody, B. S., and J. G. Dusser De Barenne 1932 Effect of hyperventilation on the excitability of the motor cortex in cats. Arch. Neur. and Psych., vol. 28, p. 571.
Dusser De Barenne, J. G., and W. S. McCulloch 1934 An ‘extinction’ phenomenon on stimulation of the cerebral cortex. Proc. Soc. Exp. Biol. and Med., vol. 32, p. 524.
Dusser De Barenne, J. G., and W. S. McCulloch 1937 Local stimulatory inactivation within the cerebral cortex, the factor for extinction. Am. J. Physiol., vol. 118, p. 510.
Dusser De Barenne, J. G., W. S. McCulloch and L. F. Nims 1937 Changes of hydrogen ion concentration of the cerebral cortex. Proc. Soc. Exp. Biol. and Med., vol. 36, p. 462.
MacInnes, D. A., and M. Dole 1929 Tests of a new type of glass electrode. J. Ind. and Eng. Chem., Analytical Ed., vol. 1, p. 57.
Hitchcock, D. I., and A. C. Taylor The standardization of hydrogen ion determinations. I. Hydrogen electrode measurements with a liquid junction. J. Am. Chem. Soc. (In press.)
Burr, H. S., C. T. Lane and L. F. Nims 1936 A vacuum tube microvoltmeter for the measurement of bioelectric phenomena. Yale J. Biol. and Med., vol. 9, p. 65.
Lehmann, J. E. 1937 The effect of changes in pH on the action of mammalian nerve fibers. Am. J. Physiol., vol. 118, p. 600.
For further research:
Wordcloud: Acid, Activity, After-Discharge, Animal, Area, Artificial, Barenne, Cerebral, Changes, Circuit, Cortex, Cortical, Curare, Decrease, Dial, Dusser, Electrical, Electrocorticogram, Electrode, Excitability, Experiment, Fig, Figure, Focus, Following, Found, Glass, Hyperventilation, Increase, Induced, Injection, Lower, Marked, Measure, Monkey, Observed, Paper, Ph, Potential, Record, Respiration, Results, Shift, Stimulation, Used, Ventilation, Voltage, Wick
Keywords: Cortex, Cat, Activity, Paper, Animal, Knowledge, Stimulation, Truth
Google Books: http://asclinks.live/nrd5
Google Scholar: http://asclinks.live/jex3
Jstor: http://asclinks.live/xirc