THE FUNCTIONAL ORGANIZATION OF THE CEREBRAL CORTEX1[57]
W.S. McCulloch
Functional organization, which defines the temporal course of activity in any nervous mechanism, depends upon both physico-chemical reactions of constituents and their anatomical relations. Since reactions of all neurons are similar, it is frequently possible to deduce anatomy from observed activity or to predict activity from known anatomy.
General organization
From a single enlargement of the neural tube, the prosencephalon, arise the cortex cerebri and the corpus striatum anteriorly and the thalamus posteriorly. At a comparatively early stage of development antero-poeterior and postero-anterior connections are established. They serve to relate certain portions of the cortex and basal ganglia (1, 2) to particular thalamic nuclei—which relay signals thither and receive them thence. Also at an early stage, there emerge from the cortex axons which, passing ventrally, make their way to efferent structures. Thus, functionally, these forebrain structures constitute a bridge of internuncials which in lower animals is but one of many. As the cortex evolves, the other bridges either disappear or become unable to mediate all sense modalities. This development correlates with increasing anatomic differentiation of the thalamic nuclei, which have few or no internuncials and, with few and small exceptions, no descending connections. Hence, as the forebrain structures become necessary for afferents of a given sense modality to affect efferent systems, the cortical or striatal link also becomes necessary, and that in two ways. It is both the internuncial system coordinating activity within and among the thalamic nuclei and the only significant path thence to the efferent system.
The obvious paralyses resulting from injuries of certain cortical regions and the motor responses to their electrical stimulation have led us to call them “motor” or “electrically excitable”; but today we know that most, if not all, regions of the cortex send axons to structures whose excitation affects the contraction of striated muscle, and that all parts of the cortex are electrically excitable. Today we would not use these properties in subdividing the cortex. The thalamic nuclear connections are discrete and serve to define “cortical sectors” consisting of cytoarchitectonically recognizable areas which preserve their topological relations—i.e., those which are not altered by stretching or folding— presumably because the formation of these thalamo-cortical connections is causally related to the local differentiation of the cortex, and because they remain despite the growth and deformation of the continuous mantle as it evolves into the convoluted cortex. Moreover, certain thalamic nuclei relay, in an orderly fashion, impulses consequent upon specific sensory excitation, and the corresponding cortical sectors exhibit this specificity and orderliness which help to define the principal rôle played by any sector and to analyze its spatial detail in relation to that of the sense organs represented upon it. Even with respect to the associational structures which become disproportionately larger in the primate cortex and have thalamic relations with nuclear, structures other than primary sensory relays—namely, parts of elaborate re-entrant circuits—the same clarification of function occurs for the same reason. Thus by conscientiously ignoring the obvious deformations, gyri and sulci, save as dubious landmarks in a particular cortex, it is possible to discuss in general the function of sectors and their constituent areas. Although the function of specific subdivisions of the sectors in other ways is various in various species, the functional homologies of these other subdivisions, insofar as they have corresponding thalamic correlates, are surprisingly orderly. This makes it possible to transfer to the cortex of a given species conclusions drawn from experiments upon other species. Since we are primates and most work has been done on the monkey, Macaca mulatta, it seems best to use figures of his cortex as a frame of reference for what follows.
A cursory glance at the gross anatomy of any mammalian brain shows that the cortex on which a thalamic nucleus projects is many times larger than that nucleus, and detailed microscopy reveals that there is great overlapping of the projections. A single thalamic nuclear element thus projects to a vastly greater cortical area. As will appear clearly later, the connections in the reverse direction spread in a similar fashion. Thus the area of cortex to which a single thalamic neuron projects serves to relate it to a large portion of its own nucleus. On this account the nucleus and its cortical sector tend to behave conceitedly in a rhythm determined by the time of transit around their reentrant path. At any part of the circuit the resultant waves affect the threshold to incoming stimuli and may be initiated or interrupted by them. Whether these waves be the envelopes of nervous impulses or merely of polarization and depolarization, they are prevented by any lesion which prevents corticothalamic reverberation. Residual activity lacks their characteristic form and frequency. Other deeper structures may contribute to the background of the thalamic excitation, and their contributions may be necessary, but the “spontaneous” electrical activity will persist in a small island of cortex when all the rest of the cortex of that sector has been removed (3)—a finding which explains why diffuse pickup fails to reveal local cortical destruction.
It goes almost without saying that these same circuits serve as internuncials to relate the activities of diverse portions of the cortical sector. That these cortico-thalamo-cortical circuits play a fundamental role in determining the organization of cortical activity is attested by the synchronizing of disturbances at two parts of the same cortical sector after severance of all direct cortico-cortical connections. A similar but more complicated re-entrant path arising from cortical efferent connections serves a similar function for even larger subdivisions of the cortex. Thus, cortico-ponto-cerebello-thalamo-cortical circuits, arising from frontal, parietal and temporal regions and playing upon the anterior portions of the ventral thalamic nuclei, affect the activity of the anterior portion of the central, or sensory, sector.
This general character of overlapping projection extends to the control of motion and makes possible use of parts whose focal cortical representation has been destroyed. If the lesion be made early enough (4), although it be large, the loss of motion is far from total and investigation of the cortex by electrical stimulation indicates that other portions of the same cortical sector have far more control of the affected muscles than can be demonstrated in the intact cortex or following a similar lesion in the adult. However, no sector has ever been shown to assume a function to which it did not contribute prior to the lesion. The corpus striatum acts in parallel with the cortex and hence complete decortication leaves a well established thalamo-striatal prosencephalic bridge of internuncials, over which afferent are connected to efferent systems so that even in primates certain motor activities may still be mediated in response to stimulation of some sense organs.
Relatively diffuse but overlapping projections upon the cortex where summation results in focal excitation make possible detailed discrimination, exceeding in sharpness of outline the distribution of excitation of sense organs (5), while interconnection of these cortical foci (6) via recursive connection through deeper structures and via direct cortico-cortical paths permits the activity of each focus at any instant to be affected by the antecedent and approximately contemporaneous activity of all other foci. The resultant efferent discharges are thus the unified consequence of the totality of these related cortical focal activities. Since activities of all parts of the cortex are related by recursive activities through peeper structures, severance of purely cortico-cortical connections can be expected to destroy only the most nearly contemporaneous relations of cortical activities at the separated foci, and so to affect adversely interpretations dependent upon them. As yet we have no behavioral test whereby we might hope to detect them. This is borne out by the lack of any discoverable loss of ability following severance of the entire corpus callosum, for this is the largest single bundle of such connections and the only one severable without any cortical destruction (7, 8).
On the other hand, overactivity of cortico-cortical connections produces a spread of disturbances over the cortex along these lines of communication. This is all too familiar in the spread of the cortical activity responsible for the march of the Jacksonian convulsion, and today there is clear evidence that for this spread of disturbance direct cortico-cortical connections are necessary (9) and sufficient (10).
Common properties
Before specifying particular thalamo-cortical projections or cortico-cortical connections, common properties should be considered, for these determine in large measure the significance of the particulars, and the interpretation of electrical or other records of cortical activity and its consequences.
Impulses entering from the subjacent white matter teach all parts of the cortex via axons which ascend to its middle layer, where they branch widely and ramify as they ascend. This axonal activity is manifest electrically by the appearance of a transient voltage in which the depth becomes negative and the surface positive to any unaffected area. This phenomenon is not prevented by rendering the neurons of the recipient cortex unresponsive by local treatment with drugs. Having ramified, the incoming disturbance excites neurons of the superficial layers, producing a local surface negativity. Under light barbiturate anesthesia this may be all that happens. Under chloralose anesthesia, and sometimes under very light barbiturate anesthesia, this surface-negative wave is propagated slowly—say 20 cm. per second—in the feltwork of the cortex, dying out (11) as it travels. Under appropriate conditions it can be elicited by weak electrical stimulation of these layers, and has been called the “superficial response.” Under extremely light anesthesia, or when augmented by dilute solutions of excitants such as strychnine or metrazol, the surface-negative, or superficial, response is followed by a surface-positive, or deep, response associated with discharge of cells in the deeper layers of the cortex, whence originate axons to remote portions of the cortex, as well as those to subcortical structures. These waves can be elicited regularly by stronger electrical stimulation and are conducted with axonal velocities. There are, then, two types of purely cortico-cortical connections relating foci, at each of which arriving disturbances ascend to the middle layer, ramify upward and descend to leave the cortex. The first is the slow, superficial, surface-negative, presumably multineuronal, or multi-synaptic, intracortical type, exaggeratedly active under chloralose; the second is the rapid, deep, surface-positive, presumably axonal, intercortical type, best seen under dial. The intracortical are as diffuse as the feltwork of the cortex, whereas the intercortical are highly specific and restricted in their distribution. Under chloralose both play significant rôles in determining the spread of electrical after-discharge; under dial, only the latter (11). These types differ also in their effect upon incoming stimuli, for impulses arriving during surface negativity fail to excite, whereas those arriving during surface positivity are facilitated,—a relation which throws some light on the effect that alpha rhythm has upon response to stimulation of the eye or of the lateral geniculate, and on the driving of alpha rhythm by visual stimulation (12, 13). Taken in conjunction with inhibition at a synapse in the case of the two neuron arc of the spinal cord (14), the relation of transcortical potentials to responsiveness to incoming stimuli suggests that inhibition is to be expected when the apical dendrite of any cell is in a region negative to its axon hillock, and facilitation when polarization is the reverse.
Weak electrical stimulation of the superficial layers of the cortex, if repeated at appropriate intervals, elicits a depth response which increases to a certain maximum determined by the duration and voltage of the stimuli. This facilitation occurs at all cortical foci and is manifest in motor response from many areas. It is presumably due to spatial summation operative over relay paths and reverberating chains of intemuncials. That it has a maximum dependent upon other parameters of stimulation is presumably due to the same limitation of responding efferent cells that produces occlusion in the case of spinal reflexes, with this difference: that in the case of spinal reflexes all cells of the pool may be excited, whereas in the case of the cortex the number that may be called into play depends upon the voltage and duration of the stimulus. Increase in voltage can always spread the stimulus to more remote portions of the pool, and increase in duration of pulse will, within limits, always fire a larger proportion of the neuronal pool reached by a given voltage. Finally, repetitive activity at frequencies of 20 or more per second eventually produces a rise in threshold of the responding neurons, so that if the stimulation be continued the response declines or even disappears. This rise in threshold is presumably the chief factor for the extinction of motor response to cortical stimulation, and undoubtedly is of paramount importance in all problems of fatigue. It is associated with the development of a polarization of the correct sign to enhance the effect, and perhaps to extend it to other neurons in the vicinity. In those cases in which stimulation of any cortical area is pushed so far as to initiate self-sustained activity—called after-discharge—another factor becomes significant, for the oxygen supply, though it may increase, is locally inadequate for the demand,
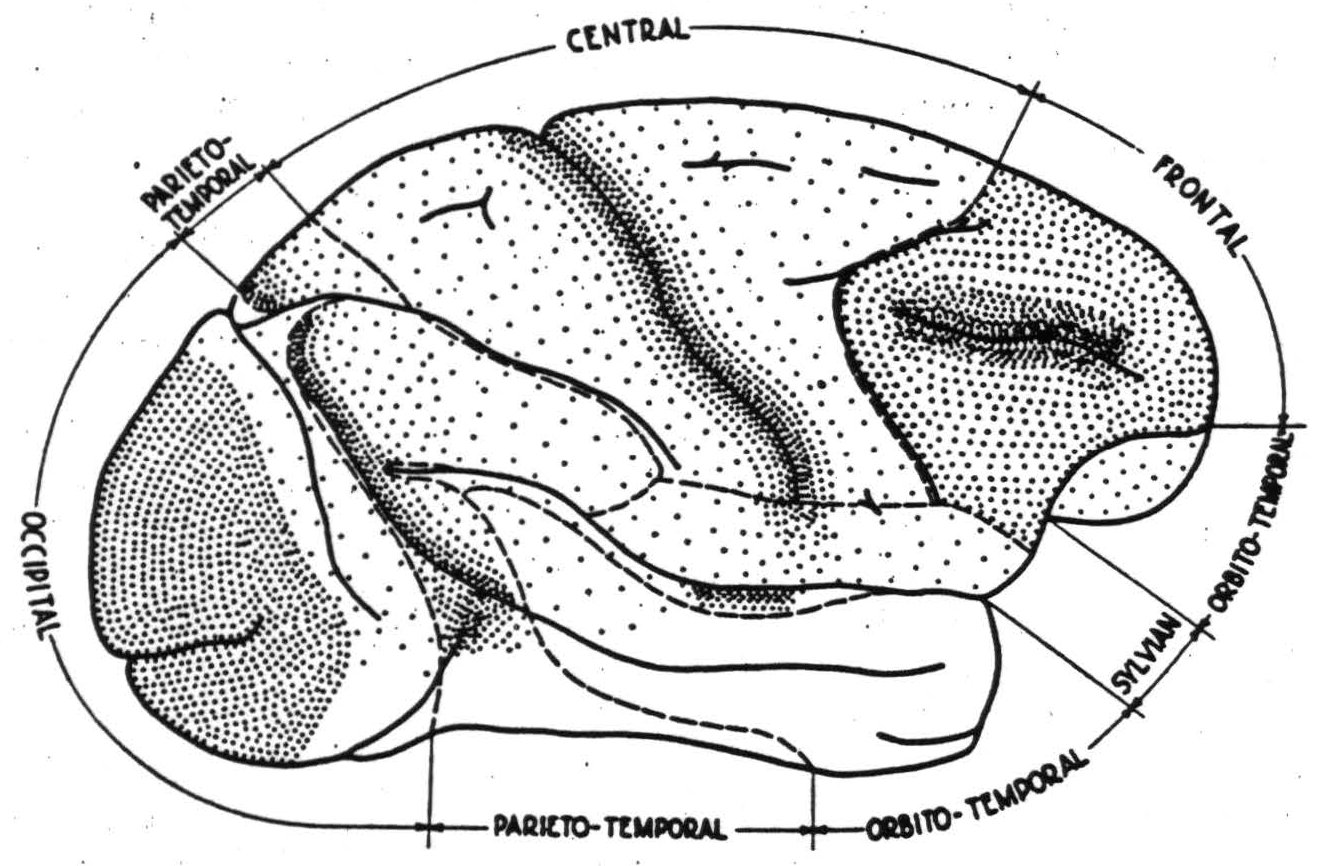
Figure 1. Macaca mulatta. Thalamic projections to cerebral cortex and division into regions described in text.
the oxygen tension falls (15) and lactic acid is produced (16). As the region becomes acid its threshold rises and eventually the self-sustained activity ceases. It may have spread over cortico-cortical connections to other regions of the cortex, but those it leaves behind are inactive and unresponsive. These are the aspects of functional organization which all parts of the cortex have in common.
Cortical sectors and regions distinguished
Thanks in no small measure to Walker's (17) and Le Grose Clark's (18) studies of retrograde degeneration of thalamic nuclei following destruction of various cortical regions, the “sectors” of the monkey's cortex defined by their thalamo-cortical connections are relatively well known. They are schematized roughly in figure 1 which also indicates the density of thalamo-cortical projection by the density of stippling. It is apparent that certain regions are dominated by these projections which define the cortical sectors.
The projection from the lateral geniculate body in primates is sharply limited to area 17, the area striata, which is surrounded by area 18, and this, in turn, by area 19, known respectively as parastriate and preoccipital. The occipital region, constituted of these three areas, has a close functional organization subserving vision.
Similarly, there is a parieto-temporal region dominated by projections from the medial pulvinar nucleus to the posterior parietal cortex and by the inferior pulvinar nucleus to the posterior temporal cortex. The whole region plays an important rôle in associative processes.
The largest single region is the central or “sensory” region, which receives impulses from the lateral thalamic nuclei. Those anterior to the central sulcus come from the ventrolateral, and those posterior from the posterolateral. The former are most dense in area 4, the latter in area 3, but as all parts of this region receive some, this sector is coextensive with this region.
Anterior to it is the frontal region whose thalamic projections arise from the dorso-medial nucleus and are most dense in a relatively small area which Walker has called area 46 for cytoarchitectural reasons. This region is granular cortex and includes all cortex anterior to the central sector on the lateral aspect, with the possible exception of area 8 which will be included in it to simplify description. On the medial side it extends just across the sulcus callosomarginalis. It does not include the opercular areas or any orbital areas proper. The latter belong to the orbitotemporal region.
The former, opercular, areas belong to the sylvian region which is dominated by the projection of the medial geniculate body to area 41 and with less density to area 42, known respectively as the primary and secondary auditory cortex and lying on the supratemporal plane, which is therefore referred to as the sector of the medial geniculate body.
The sector of the anterior thalamic nucleus lies on the posterior portion of the medial aspect of the cortex and is coextensive with the posterior limbic region, consisting of areas 23 and a small retrosplenial area which is probably best called area 29.
The thalamic projections to the remainder of the iso-cortex are unknown. This includes the anterior limbic region, or area 24, and the large orbito-temporal region which includes all the areas confined to the orbital surface of the frontal pole and the tip and greater part of the lateral aspect of the temporal lobe. These are here regarded as one region, not because there are no known thalamic projections to them but because they are related in activity. Of the remaining isocortical area, 25—and of the allocortical areas 28 and 36—we lack the knowledge to assign them to any region.
Cortical sections and regions described
Among the regions of the isocortex there are three having sectors whose activity represents sensory excitation mediated by thalamic nuclei in such a manner as to preserve topological organization—namely, the occipital, the central and the sylvian. These have been known for a relatively long time because lesions of these areas produced loss of the corresponding sensation or because excitation of the area, either in the aura of convulsive disorders or in the waking subject stimulated at operation, produced the corresponding sensation. More recently they have been investigated by stimulation of the receptor (19) and observation of the induced electrical disturbance.
Occipital region
By fixing the eye and flashing a small light in known relation to the point of fixation, while recording from electrodes in fixed positions on the occipital lobe, the visual field has been plotted on the cortex. In the primate, off and on responses are sharply restricted (20) to area 17. The point of fixation is represented on its lateral or anterior margin and only one half of the field falls on one hemisphere. Small angular displacements from the fixation point record at relatively large distances from its representation, but as the light is presented at angles increasing in equal steps the displacement per step is less on the cortex. The horizontal meridian of the visual field maps almost horizontally backward from a point about the height of the junction of the first temporal sulcus and the sylvian fissure, in the direction of the superior ramus of the calcarine fissure. The visual field represented is contralateral and inverted. It is on this area to which each retinal point projects to an appreciable circle that summation results in disproportionately sharp differentiation, so that fine eye movements combined with this summation result in discriminative ability far exceeding the analysis of the stimulus of which the retina itself is capable (5).
To investigate the connections of various cortical foci, it is possible to stimulate at one focus and record induced electrical activity propagated to remote foci. Electrical and chemical stimuli work, but the former are more difficult to control, for the current spreads to deeper structures where it excites subjacent axons, while it sets up antidromic impulses even in the gray matter. These effects are minimized by using relatively long pulses at relatively low voltages, but they cannot be entirely excluded even under optimal conditions. Hence the bulk of the work has been done with chemical stimulation. If one places a square millimeter of filter paper soaked in a saturated solution of strychnine sulfate anywhere on the cortex, the cells of the subjacent cortex send out impulses in unison. The resultant spike-like record of the transient voltage can be obtained from any point to which a sufficient number of affected cells send their axons. For brevity, we say that the strychninized focus “fires” these other points. At present there is reason to believe that strychnine poisons an acetylcholine esterase (21) and that this permits acetylcholine to accumulate, whence the cells are dischared so easily that the electrical impulse of any cell excites all the strychninized cells. In any case strychnine acts only where synapses are present on nerve cells and produces disturbances propagated only in the ordinary direction of conduction.
Local strychninization within area 17 produces these spikes but they are not propagated to points of area 17 more than about 1 mm. from the strychninized focus. At the same time there appear large spikes in the record of that portion of area 18 which is nearest to the focus. We say, therefore, that area 17 fires itself only “locally,” and fires area 18. Similar strychninization within area 18 fires an adjacent sector of area 17, fires almost all parts of area 18 of that hemisphere and the corresponding point in area 18 of the opposite hemisphere—and fires much of area 19 ipsilaterally. Thus area 17, which receives impulses in a relatively discrete fashion, keeps them so within itself but relays them to a larger fraction of area 18, which feeds excitation back into the originating segment of 17 and forward into area 19. With a relatively light Dial narcosis one frequently obtains, following both “on” and “off” effects produced by a bright light, a series of smooth, somewhat sinusoidal waves. The “on” and “off” effects are limited to area 17, but the consequent waves spread forward across area 18 to die out in area 19. Moreover, electrical stimulation of any of these areas, under appropriate conditions causes deviation of the eyes in a direction and manner dependent upon the exact site of stimulation. With ordinary 60 cycle currents one obtains from stimulation of any point in area 17 a sustained deviation which would bring foveal vision to bear on the corresponding point in the visual field (22). This form of stimulation is less effective in area 18 and the response is transient. Using pulses of rapid rising and slow falling phases, and a frequency up to about 40 per second, responses are greater and more prolonged but still not sustained. Their direction corresponds to that of the sector of area 17 to which the selected focus of 18 corresponds. Even slight stimulation of area 19 produces a relaxation of any existing muscular contraction and, in the vicinity of the intraparietal sulcus, pupillary dilatation. Moreover, stimulation of 19 will hold in abeyance the motor after-discharge, but not the cortical electrical after-discharge, set up by antecedent stimulation of other cortical foci. Finally, stimulation of this region causes a suppression of motor response to stimulation of any motor focus. This phenomenon has a latency of about four minutes, lasts from three to thirty minutes, and connot be repeated with any certainty sooner than forty-five minutes from the time of the stimulation that induced it. For brevity all three of these induced motor inactivities will be referred to as “suppression of motor functions.” There is much evidence that they do not depend on any direct or indirect cortico-cortical connections or on cortico-striatal connections, but there is no certainty as to what descending paths mediate them. In these three suppressions cortex (23) and corpus striatum (24) act in parallel fashion.
Strychninization of area 19 causes only local firing and, with a variable latency of many minutes, a diminution or complete disappearance of spontaneous electrical activity which recedes slowly across the cortex, requiring twenty to forty minutes to reach the most remote regions. Before it has reached them activity returns to the nearer regions, at first in the form of “spindles” and later in its original form.
This suppression of electrical activity, like the suppression of motor function, can be elicited from several areas—which are therefore called suppressor areas. All such areas, except 19, have been shown to project to the nucleus caudatus, and for the suppression of electrical activity by them the nucleus caudatus has been shown to be necessary. Its strychninization produces large, long voltages in the thalamic nuclei which may well break up their cortical reverberations. These presumably are not simple axonal impulses from the nucleus caudatus because of time relations and wave form. They resemble the belated and extended post-synaptic consequences of the relatively synchronous discharges of the pre-synaptic axons. In any case, when they appear in a thalamic nucleus the “spontaneous” activity of the corresponding cortical sector disappears. Until recently no trace of cortico-striatal activity could be discovered arising in area 19, but recently we have seen bumps in records from the nucleus caudatus which were definitely synchronous with strychnine spikes in area 19. Thus it, like all other suppressor areas, probably does produce its suppression of electrical activity via the nucleus caudatus. We will consider the extrinsic cortico-cortical connections of this region only after the intrinsic regional connections of other regions have been considered.
Central region
The central sector has been mapped for its most direct sensory projection by punctate stimulation exciting single points of the body surface (25) or single hairs, and locating the induced surface-positive waves on the cerebral cortex. The excited points project principally to the posterior lip of the central sulcus. The representation of the extremities occupies a disproportionately large portion of the recipient cortex. The leg is represented above a line joining the superior precentral sulcus to the superior postcentral sulcus. The arm is represented below this line to another joining the spur of the arcuate sulcus to the tip of the intraparietal sulcus. Although there is much overlapping in each of these subdivisions, the following general statements can be made. The leg subdivision begins at the sulcus callosomarginalis, which represents the tail, coccygeal segments 4 through 3; then sacral segments 3 through 1; then lumbar segments 7, 6, 5, 4; and finally, lumbar 3 through 1, overlapped by thoracic segments 12 through 1 which appear at the line of junction of the arm and leg subdivisions. Then come the cervical segments (again overlapping) in the order 2, 3, 4, 5, 6, 7, and last 8, which occupies almost the lower half of the arm subdivision. The face area below is more complicated, for the mandibular division of the 5th nerve projects above, behind and below the other two, of which the ophthalmic is above and the maxillary below. As the thalamic nuclei mediating sensibility from face, arm and leg are anatomically distinct and have been identified functionally, it is possible to subdivide the central sector along the lines described above, and to delimit these subdivisions in all primates. The anterior lip of the central sulcus receives the densest projection of the anterior portions of these same thalamic nuclei; and the motor responses elicitable by its electrical stimulation are of the somatic parts projecting to the corresponding post-central area, except at the junctions of the subdivisions where the cortex of the anterior lip is most deeply infolded. Thus the subdivision obtains for efferent as well as for afferent connections. Actually, alteration of somatic muscular contraction can be obtained from all parts of this sector under appropriate conditions, and sensory phenomena can be elicited by strychninization of any part of it, and all exhibit the same subdivisions for leg, arm and face. The conditions for obtaining motor response from points far posterior to the precentral lip are a hyperactivity of deeper structures, frequently appearing under light barbiturate anesthesia, and stimulating pulses having an abrupt rising and a 5 to 10 millisecond falling phase at a frequency of 1 to even 10 or more per second, delivered to a cortex already excited at a focus from which motor responses have been easily evoked. The response is then that elicited from the original focus. This form of so-called secondary facilitation, at least in certain instances, depends upon axons descending from both areas to the same deeper structures, rather than upon cortico-cortical connections (26). There is another form of secondary facilitation most easily demonstrated from points in the anterior portion of the central sector to points of the anterior lip of the central sulcus. Antecedent stimulation of the anterior point makes it possible to obtain the original response to excitation of the posterior point with a weaker stimulus, or to obtain a larger response with the same stimulus. This form of secondary facilitation is prevented by severance of cortico-cortical connections, and hence they presumably play some role in it.
Strychninization of a few square millimeters within any subdivision of the central sector, performed under brief anesthesia from which the animal recovers before the effects of firing of the cortex have ceased, results in the appearance of all the familiar clinical signs of paresthesia and paralgesia in the apical portions of both sides of the body belonging to the subdivision strychninized (27). The reason for the bilaterality of the reference is by no means clear, for the afferent impulses are only contralateral from the arm and leg to the thalamic nuclei and to the cortex, and the symptoms are bilateral even when the strychnine is placed on an area practically devoid of callosal connections; so that neither corticothalamic nor cortico-cortical connections seem adequate to account for the bilaterality of reference implicit in the overt behavior. That these sensory symptoms are due to thalamic rather than to cortical excitation can be deduced from this. Strychninization of a few square millimeters of the most anterior portion of leg or arm subdivision fires all of both subdivisions, but only the thalamic nucleus of the subdivision strychninized and the symptoms are referred to the corresponding members. Moreover, strychninization of the thalamic nucleus in the cat is sufficient to produce the symptoms, even when the cortex has been removed. It was on the basis of these symptoms of sensory excitation that Dusser de Barenne outlined the “sensory cortex” and subdivided it according to the part of the body subserved—leg, arm or face. It was his hypothesis that the symptoms were to be explained by projection from every part of any subdivision to all parts of its thalamic nucleus. That hypothesis has been amply substantiated by recording the electrical activity of each thalamic nucleus during strychninization of each of the constituent areas of each subdivision. His “sensory cortex” consisted of all those areas which Brodmann numbered 1 through 7 in his map of the monkey's brain. Two difficulties have arisen with respect to this sector. The lower margin of the face subdivision has proved to be higher on the frontal and parietal operculum than was originally supposed, and the method of local strychninization and recording of resultant electrical activity, which he called physiological neuronography, has compelled distinctions between parts of the cortex histologically indistinguishable by present methods. To meet these difficulties and to indicate areas which have been identified cytoarchitectonically or physiologically since Brodmann's maps, we made figure 2, a, b and c. (Hereafter numerals will be used to designate areas indicated by them
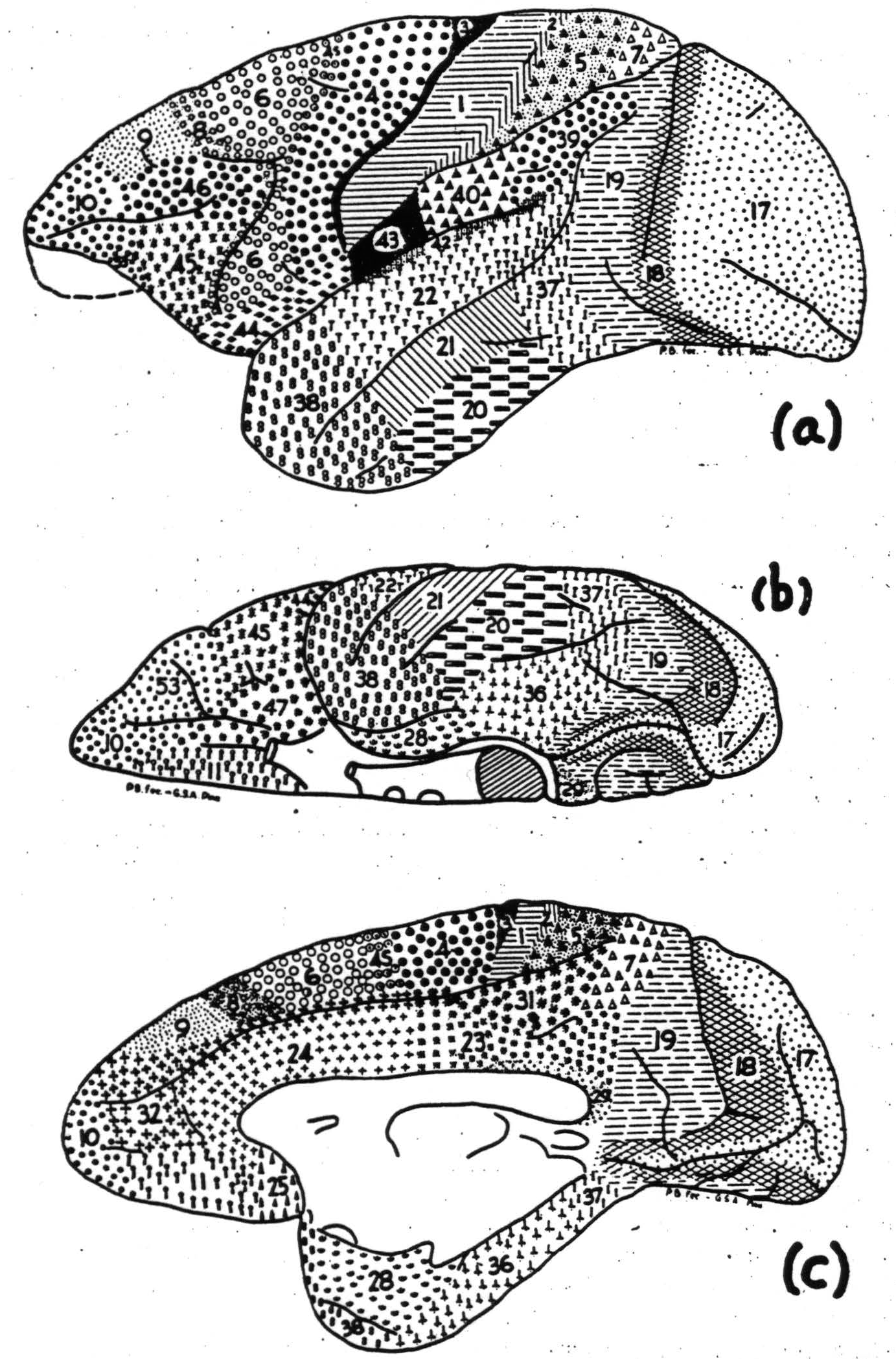
Figure 2. Macaca mulatta. Areas of cortex distinguishable by cytoarchitectonics or physiological neuronography: (a) lateral, (b) orbital and (c) medial surface.
in this figure.) The central sector, or “sensory cortex,” consists of the areas 6, 4s, 4, 3, 1, 2, 5. In the monkey, 7 and both 40 and 39 (of whose cytoarchitectural identity with area 7 Brodmann was himself uncertain) are all sensory in Dusser de Barenne's sense of the term, and both fire the postero-lateral thalamic nuclei but poorly. In the chimpanzee, 40 and 39 fail to fire it. In both primates they fire heavily into the pulvinar. Therefore, although their principal cortico-cortical connections are with the central sector, they must be considered as belonging to the parieto-temporal region, which is dominated by the sector of the pulvinar. Excluding these and the opercular areas, the firing from one to another of the component areas can be stated briefly; 6, leg or arm, fires all component areas of both subdivisions; 4 fires itself, 1, 2 and 5; only in the face subdivision does it fire face 6; 1, arm and leg, each fires itself locally, and the corresponding 4 and 5 well, whereas 1 face fires 6 face and 4 face; 5, arm and leg, each fires itself more widely, and the corresponding 4, 1, 2 and 7; 4s and 2 fire themselves locally and suppress the electrical activity of the cortex generally. They also suppress motor activity. To this region neither 44 nor 43 properly belong, although there is evidence that taste may be projected upon 43 (28) and although motion of the tongue and larynx are obtained from 44 by primary stimulation and from 43 by secondary facilitation.
Sylvian region
For descriptive purposes, 43 and 44, although firing face 6, are considered part of the sylvian region, which is dominated by projection from the medial geniculate body to a slight elevation, corresponding to Heschl's gyrus, running from near the middle of the lower lip of the sylvian fissure diagonally upward and inward. This corresponds to the primary auditory cortex of the cat, upon which the organ of Corti maps point for point. The arrangement is exactly what is to be expected when the twist of the region from cat to monkey is kept in mind (29). Lower tones are represented on its anterior or lateral end, and higher tones more posteriorly or medially. Still farther posteriorly, extending to the end of the sylvian fissure and sometimes emerging into the parietal region in positions corresponding to Campbell's “audito-psychic” area in the human brain, is what is probably best called the secondary auditory cortex, resembling the corresponding area in the cat. The “primary” area, 41, hidden in the sylvian fissure, is koniocortex, and the “secondary,” 42, the para-acoustic area, contains very large pyramidal cells in layer iii. These areas are difficult to expose. Even subpial resection of the parietal operculum leaves them in a condition which makes truly local strychninization difficult. At present it seems best to confine remarks to the following statements: 41 fires 42; 42 fires itself well, and fires 41, as well as adjacent portions of 22. The posterior part of the superior temporal convolution fires area 42, but it is uncertain whether this is referable to strychninization of 22 or 37. No definite relation to any of the posterior parietal areas has been established, although in the cat partial asphyxia will cause a response to a click to appear in them synchronously with the response of the auditory cortex. The requisite opercular destruction prevents study of the connections of one to the other beneath the sylvian fissure, except from the surfaces exposed in the intact cortex. These have shown the following connections: 43 fires 44 and 22; 22 fires 44 and 43 and probably 42. Thus this region contains structures necessary for vocal response to auditory stimulation, and their cortico-cortical connections are direct.
Parieto-temporal region
The region lying between the occipital posteriorly and the central and sylvian anteriorly, is roughly triangular with an extension forward on the medial aspect, whence it extends to a wide base along the inferior aspect of the temporal lobe. It includes 31 and probably 7 on the medial aspect, and 39, 40, 37, 20 on the convexity of the hemisphere. It is dominated by areas 39 and 37 which receive the bulk of the pulvinar projections. Each of its constituent areas fires itself well but its inter-areal connections are relatively few or restricted. 31 fires none of the other constituents. The line between 39 and 40 is vague, and these areas may fire each other, but only from neighboring portions. The upper limit of 37 is equally vague, and 37 fires 39. Similarly, more work is necessary to bound 37 anteriorly, but there is nothing to suggest that it fires or is fired by areas anterior to it. Per contra, its interregional relations are obvious and important, as will appear later.
Limbic regions
The posterior limbic region, or sector of the anterior nucleus (30), lying between 31 and the corpus callosum, is made of two areas: one, which fires only itself and that only locally, called 29 for histological reasons; the other, 23, which fires itself throughout. The anterior limbic region is a single suppressor area, 24, which, like other suppressor areas, fires itself and only locally. Of areas near its anterior end—i.e., 25 and 11, we lack information and hence are unable as yet to assign them to this region or to the orbito-temporal or frontal regions.
Frontal region
The frontal region is bounded posteriorly by the central, medially by the anterior limbic and orbitally by 11, 47 and 53. In the monkey this region has not been clearly analyzable by local strychninization and distribution of firing within the region, except for two areas: 32, which fires itself throughout but no other frontal area, and 8, which is a suppressor area and fires itself extremely locally and fires into 32. Moreover, area 8 is “motor” for contralateral, and anterosuperiorly for ipsilateral, eye movements associated with dilatation of the pupil. This dilatation, in the case of the cat, is due to inhibition of the third nerve nucleus (31). As of eye movements obtained from 17, 18 and 19, it is possible to conclude that the cells of origin for the requisite structure lie in the area in question. Eye movements are obtainable from the parietal cortex generally, if short, high voltage pulses are used. These can stimulate underlying white matter, and thermocoagulation of the parietal cortex does not prevent them from being elicited, whereas it abolishes those evoked from 8. Unilateral destruction of 8 produces a pseudo-hemianopia (32) and circus movements. Immediately anterior to its central third lies 46, which receives the densest projection from the dorso-medial nucleus. Its strychninization results in relatively wide firing of the frontal pole. Anterior to it is 10 where firing is most restricted. The cortex medial, or dorsal, to area 46, being bounded by areas that can be thus defined, is called 9. The area ventral, or lateral, to 46—i.e., 45, is distinguished by its firing 44 and 40.
Orbito-temporal region.
The remainder of the isocortex, characterized by deficit of thalamic connections, constitutes the orbitotemporal region. The orbital areas, 47 and 53, are easily distinguishable, for electrical stimulation of 47, but not of 53, causes an arrest of respiration in inspiration with the vocal cords abducted—as in a yawn. Moreover, they fail to fire each other and are cytoarchitecturally distinct, for 47 is dysgranular and 53 is cugranular. The temporal areas 38, 21 and 22 are difficult to distinguish histologically in the monkey. Each fires itself throughout but fails to fire the others. The relation of the orbital to the temporal components is as follows: 47 fires 38; 53 fires 37; 38 fires 47; 22 fires 53. It seems safe to assume that these connections form the bulk of the fasciculus uncinatus.
Intracortical connections
While we have been considering only the inter-cortical connections as revealed by strychninization under dial narcosis, it must be remembered that the belt of intra-cortical fibers exists and under other conditions can be shown to relate relatively distant foci. As these connections run in all directions, they relate areas regardless of the regions or sectors to which they belong. They cannot, however, relate an area to a distant one without crossing those areas which surround the excited area. This is strictly a topological problem in two dimensions. It is, therefore, invariant under continuous deformation of the cortex. Hence it is possible to map the entire cortex and the basal ganglia on a plane without losing the relations determined by the intracortical connections. Figure 3 is such a map. In it the margins of the cortex of one hemisphere appear stretched around the periphery, and the adjacent areas are consequently greatly elongated. Moreover, all the sulci are treated as if they were opened out. This, for example, discloses area 3 and the continuity of area 6 from arm to face subdivisions, and brings to the surface the insular cortex. In so doing it makes clearer the lack of information. The basal ganglia have been so displaced as to make them visible without severing their continuity with cortical structures either anteriorly, where 47 is continuous with the putamen, or posteriorly, where the amygdaloid nucleus adjoins the allocortex of the temporal lobe.
Inter-regional connections
The connections between the occipital and parieto-temporal regions probably constitute both the fasciculus longitudinalis inferior and the fasciculus occipitalis verticalis (of Wernicke), and the long antero-posterior connections, the superior longitudinal fasciculus. Just at the time that the connection of all suppressor areas to the belt-like areas 31, 32, were discovered by physiological neuronography, the connection of 4s to this same band was followed by myelin stain (33). Most of the remaining connections as yet lack correspondingly precise anatomical footing. Since the corpus striatum not only arises in parallel with the cerebral cortex, but also acts in parallel with it, at least in suppression of motor activity, the cortico-striatal connections established by physiological means are here included. Areas 24, 8, 4s, 2 and probably 19 all fire the nucleus caudatus, whereas 6 fires the putamen and the external segment of the globus pallidus, and 4 fires the putamen but not the external segment of the globus pallidus. Recently, by a new method applied to the cat's brain, connections from some suppressor areas to the nucleus caudatus have been shown to be by fine nonmyelinated collaterals from descending axons (34). This accounts for previous failures to find them by Marchi studies of degeneration (35).
Inter-hemispherical connections
For the most extensive anatomical studies of the connections of the two hemispheres we are indebted to Mettler (36, 37, 38, 39), according to whom almost all parts of one hemisphere send
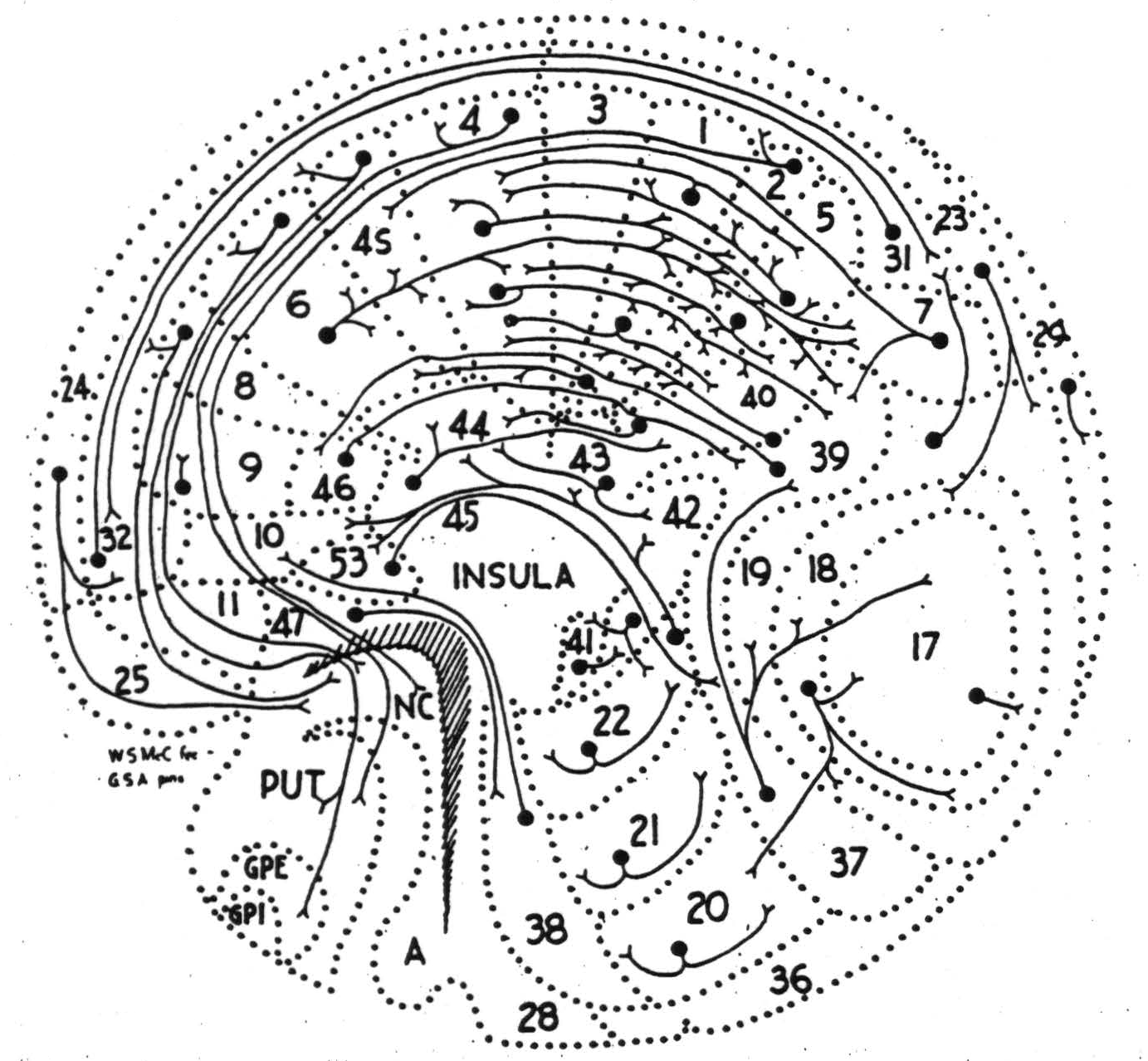
Figure 3. Macaca mulatta. Development of cortex and basal ganglia preserving topological relations. On it are indicated all cortico-cortical and cortico-striatal connections demonstrated by physiological neuronography.
axons to a corresponding but larger and related portion of the other, while certain regions send them to many portions of the opposite hemisphere—so-called heterotopic connections. Possibly because the lesions affected the underlying white matter, the projections described by him are more extensive than those found by electrical stimulation, although this may have excited certain of the more superficial fibers. The reason for the difference may also be that the discovery of the electrical disturbance on the receiving hemisphere depends necessarily upon the synchronous activity of enough axonal endings in a small space. A diffuse projection might easily be missed. This applies also to physiological neuronography, with this difference: that the strychnine can excite only neurons, not axons or axonal terminations. Hence its findings can only be of fewer projections than by these other means. Figure 4 schematizes the origin of the commissural connections as revealed by physiological neuronography. It discloses the direction as well as the most concentrated axonal distributions rather than their totality.
Mettler used the term “homoiotopic” for the more diffuse anatomical projections to corresponding related portions of the contralateral hemisphere. These are always restricted to constituent areas of the same contralateral region. Even what he has called “heterotopic” connections rarely exceed these limits.
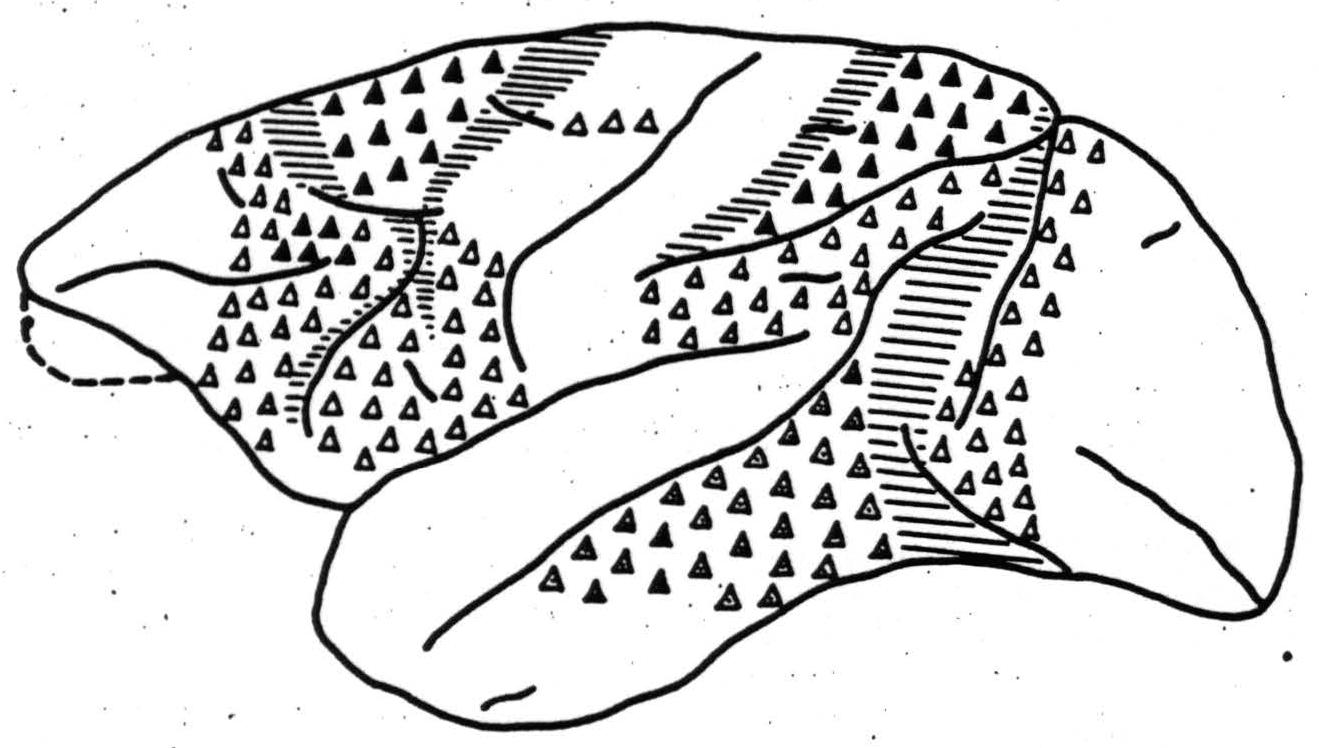
Figure 4. Macaca mulatta. Cortical origin of commissural connections ▲, to widely separated points; △, to symmetrical point only and stopped by section of corpus callosum; ◬, to symmetrical point only but persisting though diminished after section of corpus callosum.
When they do so the exceptions conform to the interregional connections of the homolateral projections. There is an additional reason, therefore, for inquiring into the latter in some detail.
Remaining difficulties
In the first place, the study of the interregional connections has not been pushed as hard as the studies of the functional organization of the regions themselves, and there may be many connections as yet undiscovered, but the connections so far revealed are so numerous and intricate as to make any regional subdivision seem somewhat arbitrary. This is particularly true with respect to areas 31 and 32, which constitute a narrow band along the sulcus calloso-marginalis, with extensions at its anterior and posterior extremities. The extremities are clearly different, histologically, but in the intermediate region both areas shade into the adjacent areas of the central region so that no definite boundaries can lie established. Moreover, this band is internally connected by axons from any part to all parts in the monkey, whereas in the chimpanzee the connections are by no means as strong to distant portions. In the monkey, all parts of the band are fired from all suppressor areas, whereas in the chimpanzee the firing is more restricted. To assign different portions of this band to different regions seems arbitrary in spite of the dissimilarity of thalamic connections. A similar difficulty occurs with 7, 39 and 40, which are unmistakably sensory (as that term was used by Dusser de Barenne). Moreover, they have powerful connections to and from the rest of the central sector despite their projections to and from the pulvinar. The entire parieto-temporal region is an extreme example of long, interregional connections, for there is no region on the convexity of the hemisphere with which it fails to make connections in both directions. Inferentially, it subserves associational functions of a high order. The frontal region obviously has not differentiated as far in the monkey as in the chimpanzee, nor has it as extensive connections with the rest of the hemisphere. The sylvian region containing the projection areas for sound and the control of respiration, lingual and laryngeal musculature, and the interconnections of these areas, is obviously the precursor of those cortical structures which in man are principally responsible for speech. Unfortunately, they are hidden in the sylvian fissure to such an extent that their connections are difficult to investigate; but it is clear that they are in part, at least, recipients of impulses from the portion of the temporoparietal region from which the angular and supramarginal gyri of man arise. The behavioral implications are self-apparent, for there do not exist any connections in the thalamus whereby these areas could be related once the cortex is destroyed. Comparison of the newest maps of interregional connection with antecedent studies indicates how easily these have been missed, and a recognition that that part of the cortex lying in sulci has never been investigated implies that many more are still to be expected. Obviously, no conclusions can be drawn from the apparent lack of connections of particular regions. Only one general statement concerning these connections seems to hold: namely, that the primary sensory areas do not give rise to interregional associational fibers. This again emphasizes the importance of the “associational areas.” It is apparent today that certain tenets of the psychology of perception can be deduced from the physiology of the primary sensory areas. In similar fashion we have every right to expect that utimately knowledge of “associational areas” and of interregional connections will lead to a physiological interpretation of the more complex psychological problems.
In order to bring this article (which is essentially a review) as nearly up to date as possible, I have taken the liberty of including hitherto unpublished work done in collaboration with G. von Bonin, H. W. Garol, E. W. Davis, A. Silveira and P. Bailey—to the last of whom we are indebted for figure 2.
Footnotes
References
Ranson, S. W., S. W. Ranson, Jr. and M. Ranson. Arch. Neurol. and Psychiat. 46: 230, 1941.
Ranson, S. W., S. W. Ranson, Jr. and M. Ranson. Arch. Neurol, and Psychiat. 46: 401, 1941.
Dusser De Barenne, J. G., and W. S. McCulloch. J. Neurophysiology 4: 304, 1941.
Kennard, M. A. and W. S. McCulloch. J. Neurophysiology 6: 181, 1943.
Marshall, W. H. and S. A. Talbot. Biol. Symposia 7: 117, 1942.
Dusser De Barenne, J. G. and W. S. McCulloch. J. Neurophysiology 2: 319, 1939.
Akelaitis, A. J. Arch. Neurol and Psychiat. 48: 914, 1942.
Van Wagenen, W. P. and R. Y. Herren. Arch. Neurol, and Psychiat. 44: 740, 1940.
Erickson, T. E. Arch. Neurol. and Psychiat. 43: 429, 1940.
Dusser De Barenne, J. G. and W. S. McCulloch. J. Neurophysiology 1: 69, 1938.
Rosenblueth, A. and W. B. Cannon. Am. J. Physiol. 136: 690, 1942.
Bartley, S. H. J. Cell. and Comp. Physiol. 8: 41, 1936.
Walker, A. E., J. I. Woolf, W. C. Halstead and T. J. Case. J. Neurophysiology 6: 213, 1939.
Lloyd, D. P. C. J. Neurophysiology 4: 184, 1941.
Davis, E. W., W. S. McCulloch and E. Roseman. (As yet unpublished.)
Stone, W. E. Personal communication.
Walker, A. E. The primate thalamus. University of Chicago Press (1938).
Clark, W. E. Le Gros. Brain 55: 404, 1942.
Woolsey, C. N., W. H. Marshall and P. Bard. J. Neurophysiology 6: 287, 1943.
von Bonin, G., H. W. Carol and W. S. McCulloch. Biol. Symposia 7: 165, 1942.
Nachmansohn, D. Personal communication.
Walker, A. E. and T. A. Weaver, Jr. J. Neurophysiology 3: 353, 1940.
Dusser De Barenne, J. G., H. Garol and W. S. McCulloch. Association for Research in Nervous and Mental Disease 21: 246, 1942.
Mettler, F. A., H. W. Ades, E. Lipman and E. A. Culler. Arch. Neurol and Psychiat. 41: 984, 1939.
Woolsey, C. N., W. H. Marshall and P. Bard. Bull. Johns Hopkins Hosp. 70: 399, 1942.
McCulloch, W. S. Cortico-cortical connections of the pre-central motor cortex. Chapter 8, in P. C. Bucy. The precentral motor cortex. Urbana, University of Illinois Press, 1944.
Dusser De Barenne, J. G. Proc. Roy. Soc. (London), 96B: 272, 1924.
Bӧrnstein;, W. S. Am. J. Physiol. 129: 314, 1940.
Bailey, P., G. Von Bonin, H. Garol and W. S. McCulloch. J. Neurophysiology 6: 121, 1943.
Walker, A. E. J. Comp. Neurol. 64: 1, 1936.
Hodes, R. And H. W. Magoun. J. Comp. Neurol. 76: 461, 1942.
Kennard, M. and L. Ectors. J. Neurophysiology 1: 45, 1938.
Walker, A. E. The afferent connections of the precentral motor cortex. Chapter 4, in P. C. Bucy. The precentral motor cortex. Urbana, University of Illinois Press, 1944.
Glees, P. Personal communication. See also J. Anat. 78: 47, 1944.
Verhaart, W. J. C. and M. A. Kennard. J. Anatomy 74: 239, 1940.
Mettler, F. A. J. Comp. Neurol. 61: 221, 1935.
Mettler, F. A. J. Comp. Neurol. 61: 509, 1935.
Mettler, F. A. J. Comp. Neurol. 62: 263, 1935.
Mettler, F. A. J. Comp. Neurol. 63: 25, 1935-36.
For further research:
Wordcloud: Activity, Anterior, Area, Arm, Axons, Cells, Central, Connections, Corresponding, Cortex, Cortical, Cortico-Cortical, Direct, Electrical, Excitation, Face, Figure, Fires, Following, Function, Impulses, Lateral, Leg, Local, Medial, Motor, Neurol, Nuclei, Nucleus, Parts, Physiological, Point, Portions, Possible, Posterior, Produces, Projections, Region, Related, Relatively, Response, Sector, Sensory, Stimulation, Structures, Strychninization, Subdivision, Sulcus, Sylvian, Thalamic
Keywords: Cortex, Activity, Organization, Looms, Area, Constituents, Neurons, Cortico-Cortical, Nucleus
Google Books: http://asclinks.live/0fn2
Google Scholar: http://asclinks.live/l9fs
Jstor: http://asclinks.live/vsad