MOTOR RESPONSE TO STIMULATION OF CEREBRAL CORTEX IN ABSENCE OF AREAS 4 AND 6 (MACACA MULATTA)12 [50]
M.A. Kennard and W.S. McCulloch
Previous experiments have established two facts with regard to the influence of the cerebral cortex on motor performance of monkeys: (i) Ablation of the motor areas (areas 4 and 6 of Brodmann) in infancy has less effect than removal of the same areas from older animals; (ii) removal of the frontal areas (areas 8-12) or of the postcentral gyrus (areas 3, 1, 2) from normal animals does not cause paresis, but, if areas 4 and 6 have been previously removed in infancy, subsequent extirpation of either frontal or post-central regions is followed by a marked increase in motor deficit (1).
Some reorganization of function of these “non-motor” regions must thus occur after areas 4 and 6 have been removed in infancy. Since these altered areas might then become more excitable to cortical stimulation, the cortices of five nearly adult monkeys have herewith been stimulated following motor ablations made at a much earlier date. Four of these animals had been operated upon in infancy and the excitability of their cortices explored more than two years thereafter. The fifth, used as control, was nearly mature and had been operated upon five months previously. In all, apparently maximal recovery of function had taken place some time before the terminal experiment.
Method
The five animals were all Macaca mulatta. The four operated upon in infancy were born in the colony and of known age. The fifth was much older, a nearly mature male weighing 4.2 kg. All cortical ablations were made by the same individual and with the same technique. Areas 4 and 6 were totally removed from one or both hemispheres of four monkeys. The lesion extended to the depth of the cingulate gyrus on the medial aspect and through the face area laterally. It is probable that all of area 6b was not removed in every case, since its margins are indefinite and vary with the shape of the arcuate sulcus. The extirpation was taken to the depth of the central sulcus. The fifth animal had bilateral extirpation of areas 4, 3-1-2 comprising the pre- and postcentral gyri. Area 6 remained intact.
It was necessary in the case of the control animal to use a preparation with a unilateral ablation because older animals with bilateral ablations are so severely paralyzed that they are difficult to keep alive, and, it was felt that a preparation of long-standing to insure maximal recovery was essential for comparison with those of infants showing maximal recovery after bilateral ablations. At the time of sacrifice, the cortices were exposed under dial anesthesia (0.6 cc. per kg. given one-half intraperitoneally and one-half intramuscularly). Stimulation was with bipolar electrodes by a current controlled for wave form, frequency and voltage. After removal of brain at autopsy, sections were made and stained by Nissl technique so that the extent of the lesions might be verified histologically.
ablation of left areas 4 and 6 Sept. 30, 1939; ablation of right areas 4 and 6 Oct. 30, 1939; cord transection at Th. 6 Jan. 2, 1942.
Following the cortical ablations during the 6th and 7th months of life this animal recovered sufficient motor skill so that it was able to feed and carry on ordinary cage activities. It had, however, marked scissors gait in the hind legs and used fingers poorly in voluntary prehension. Resistance to passive manipulation was moderately increased and tendon reflexes were markedly hyperactive. For another purpose the cord had been transected in the mid-thoracic region prior to terminal experiment. Subsequent stimulation on the cortex was consequently valid only in those regions supplying upper extremities or face.
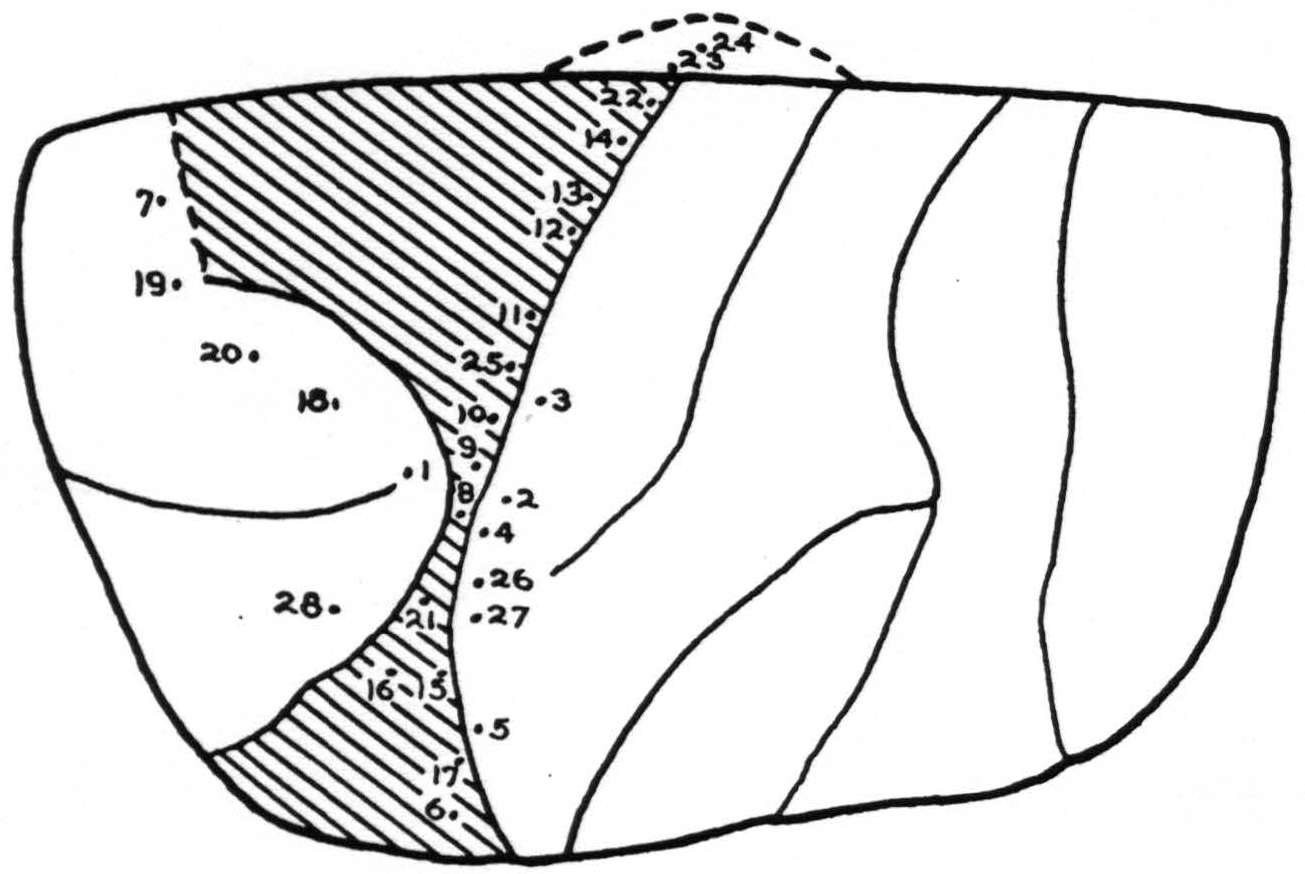
Stimulation: In Fig. 2, the points stimulated on the left cortex are shown. No response was obtained at any point except the following:
| Point | Response |
|---|---|
| 8 | Tongue withdrawn |
| 9 | Tongue withdrawn, movement of lower lip |
| 10 | Tongue, Shoulder and hand movement |
| 12 | Tongue, shoulder and arm |
| 13 | Tongue withdrawn, fingers flexed |
| 14 | Fingers, upper lip |
| 15 | Tongue |
| 16 | Protrusion of tongue |
| 19 | Eyes turned rt. |
| 21 | Tongue, torsion |
| 22 | Upper lip |
| 25 | Tongue |
Summary: Normal characteristic movements of lids and eyes resulted from stimulation of area 8. Discrete rapid movements of the lip were obtained with a stimulus of the type usually effective in area 4, from points 14 and 22. Slow generalized movements of the fingers appeared from stimulation of points 12 and 13. They were usually accompanied by tongue and lip movements. There were no discrete finger movements but instead slow diffuse flexion of fingers and wrist, with occasional spread to forearm. Movements of the tongue and lip were obtained from points 8, 9, and 10 easily and quickly and with low voltages. Higher voltages caused the same movement at longer latency from any spot in the entire scar. This was obviously due to spread of current via the scar tissue. Movements of arm and face were elicited from the entire length of the posterior lip of the central sulcus.
Experiment 3 (I 50) July 17, 1942. A male, 2 years, weight 2.1 kg. Born Jan. 8, 1940. Bilateral ablation of areas 4 and 6 Jan. 30, 1940.
Simultaneous bilateral removal of areas 4 and 6 produced a deficit in this infant which was similar in kind but slightly more severe than that of the animal in Expt. 2. There was marked crossing of the hind limbs in walking, increased resistance to passive manipulation and active tendon reflexes. Voluntary prehension was almost impossible, but climbing and clinging were well executed.
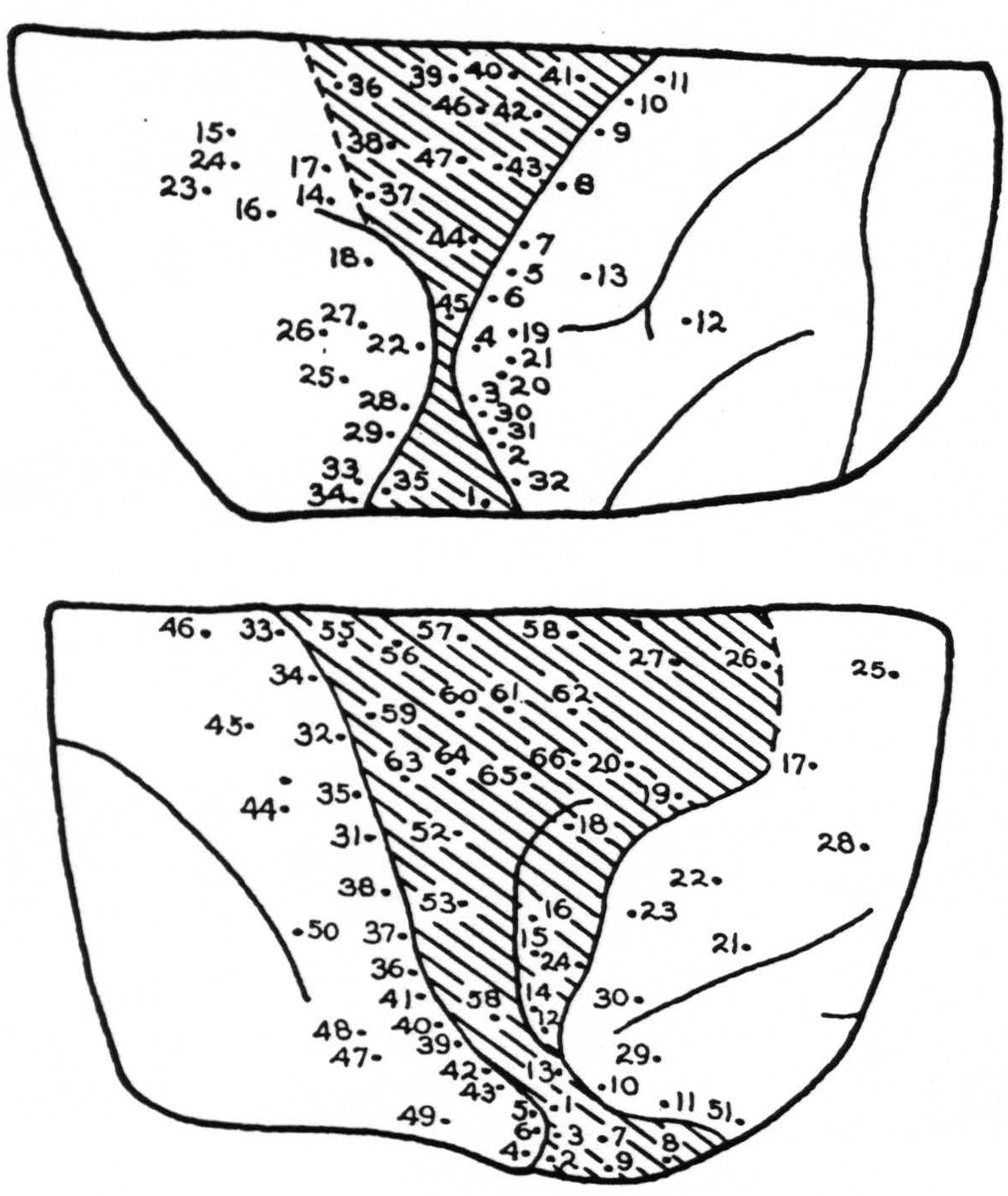
Stimulation: In Fig. 3, A and B, the points stimulated on the left and right cortex respectively are indicated numerically. No response was obtained except from the following points:
| Lt. Cortex | |
|---|---|
| Point | Response |
| 5. | Arm, wrist extension |
| 7. | Arm, more marked |
| 8. | Elbow |
| 9. | Diffuse arm |
| 10. | Elbow and wrist, eversion |
| 11. | Elbow'and wrist, eversion |
| 14. | Deviation of eyes rt. |
| 15. | Convergence of eyes |
| 16. | Lids twitch, pupils dilate |
| 17. | Lids, followed by arm |
| 18. | Shoulder, after long latency |
| 19. | Wrist and elbow |
| 21. | Extension of wrist |
| 22. | Upper arm and face |
| 23. | Eyes turned to lt. |
| 25. | Lt. eye turned rt. |
| 27. | Rt. shoulder |
| 28. | Upper lip, slight |
| 30. | Extension of wrist, with after-discharge |
| 31. | Wrist extension, followed by elbow |
| 32. | Upper lip, then arm |
| 33. | Rt. eye to lt. |
| 34. | Convergence, pupils dilate spread to lower jaw |
| 35. | Upper lip |
| 37. | Diffuse shoulder |
| 38. | Upper lip, shoulder, elbow |
| 41. | Wrist extension |
| 43. | Wrist |
| 44. | Wrist and elbow |
| 46. | Pronation |
| 47. | Flexion of index finger, then wrist |
| Rt. Cortex | |
|---|---|
| Point | Response |
| 1. | Face |
| 2. | Face |
| 3. | Face |
| 5. | Face |
| 6. | Face |
| 8. | Face |
| 9. | Face |
| 10. | Lids opened |
| 13. | Eyes to It. slight |
| 15. | Eyes to It. |
| 16. | Eyes to It. slowly |
| 17. | Eyes to rt. |
| 18. | Eyes to It. lids open, pupils dilate |
| 19. | Lids open, then close |
| 20. | Adduction and flexion of forearms |
| 25. | Vertical nystagmus, lachrymation |
| 26. | Lids open, eyes move down, homolateral arm moved |
| 27. | Convergence of eyes, flexion of rt. extension of It. arm |
| 31. | Extension of wrist |
| 32. | Extension of wrist |
| 33. | Internal rotation, shoulder |
| 34. | Pronation, wrist |
| 37. | Abduction, thumb |
| 47. | Extension of wrist, then thumb |
| 51. | Eyes opened, chewing |
| 52. | Extension wrist, then supination |
| 53. | Abduction, shoulder |
| 59. | Abduction, wrist 60 |
| 60. | Extension and eversion, wrist |
| 61. | Shoulder and elbow |
| 63. | Face and neck |
| 64. | Thumb adducted |
| 66. | Eversion and flexion wrist, some eye movements |
Summary: From the intact area 8 characteristic eye movements were elicited. From stimulation the depth of the rostral portion of the scar, diffuse slight movements which were of the type elicited from area 6 of an intact hemisphere, appeared. Movements of hands, wrists and forearms were obtained from the posterior lip of the central sulcus in areas 3 and 1. Strychnine placed in an excitable point within the scar did not increase excitability. This was taken to indicate that excitability at this point was due to spread on scar tissue and not to active cells within this area.
Experiment 4 (I 60) July 18, 1942. Male, 2 years, weight 2.3 kg. Born Apr. 17, 1940. Ablation of left areas 4 and 6, May 6, 1940; of right areas 4 and 6 Sept. 20,1940.
The motor deficit of this animal during the year before the terminal experiment was stationary and of the same order as that of the animal in Expt. 3 but not as extreme. This last was because the ablations were seriatim and not simultaneous. There was moderate
 spasticity and marked crossing of the hind legs in walking. The forelimbs were everted, the hands and fingers flared widely. Fingers were used awkwardly for voluntary prehension.
spasticity and marked crossing of the hind legs in walking. The forelimbs were everted, the hands and fingers flared widely. Fingers were used awkwardly for voluntary prehension.
Figure 3.
Stimulation: Figure 4 shows the points stimulated in both left and right sides. Those from which responses were obtained were as follows:
| Lt. Cortex | |
|---|---|
| Point | Response |
| 2. | Face |
| 3. | Face |
| 8. | Face |
| 9. | Face, then wrist and extension, finger |
| 10. | Extension, fingers |
| 11. | Thumb extension, then wrist |
| 13. | Rt. upper lip |
| 18. | Rt. upper lip |
| 28. | Supination of wrist |
| 29. | Adduction at shoulder |
| 30. | Extension of wrist, finger, then lip |
| 31. | Lip followed by hand |
| 32. | Lip |
| 33. | Extension of wrist |
| 34. | Lip, slight |
| 43. | Eyes to rt. |
| 44. | Eyes to rt. |
| 51. | Dil. of pupils |
| 58. | Eyes moved to center |
| 63. | Eyes rt. and up; lachrymation |
| Rt. Cortex | |
|---|---|
| Point | Response |
| 10. | Hip and shoulder |
| 11. | Leg, tail and shoulder |
| 12. | Extension of forearm |
| 15. | Extension, wrist and fingers |
| 16. | Flexion, elbow, then hand |
| 17. | Eversion forearm |
| 18. | Inward rotation, shoulder |
| 19. | Inward rotation, shoulder |
| 20. | Inward rotation, shoulder |
| 41. | Eyes It. |
| 42. | Eyes It. |
| 43. | Eyes It. and up, pupils dil. |
| 44. | Eyes down, rt. pupils dil. |
| 45. | Convergence |
| 46. | Lids blink |
| 50. | Convergence of eyes, slight |
| 56. | Eyes to It. |
| 57. | Eyes to rt. |
| 59. | Eyes It. pupils dil. |
| 60. | Eyes to It. |
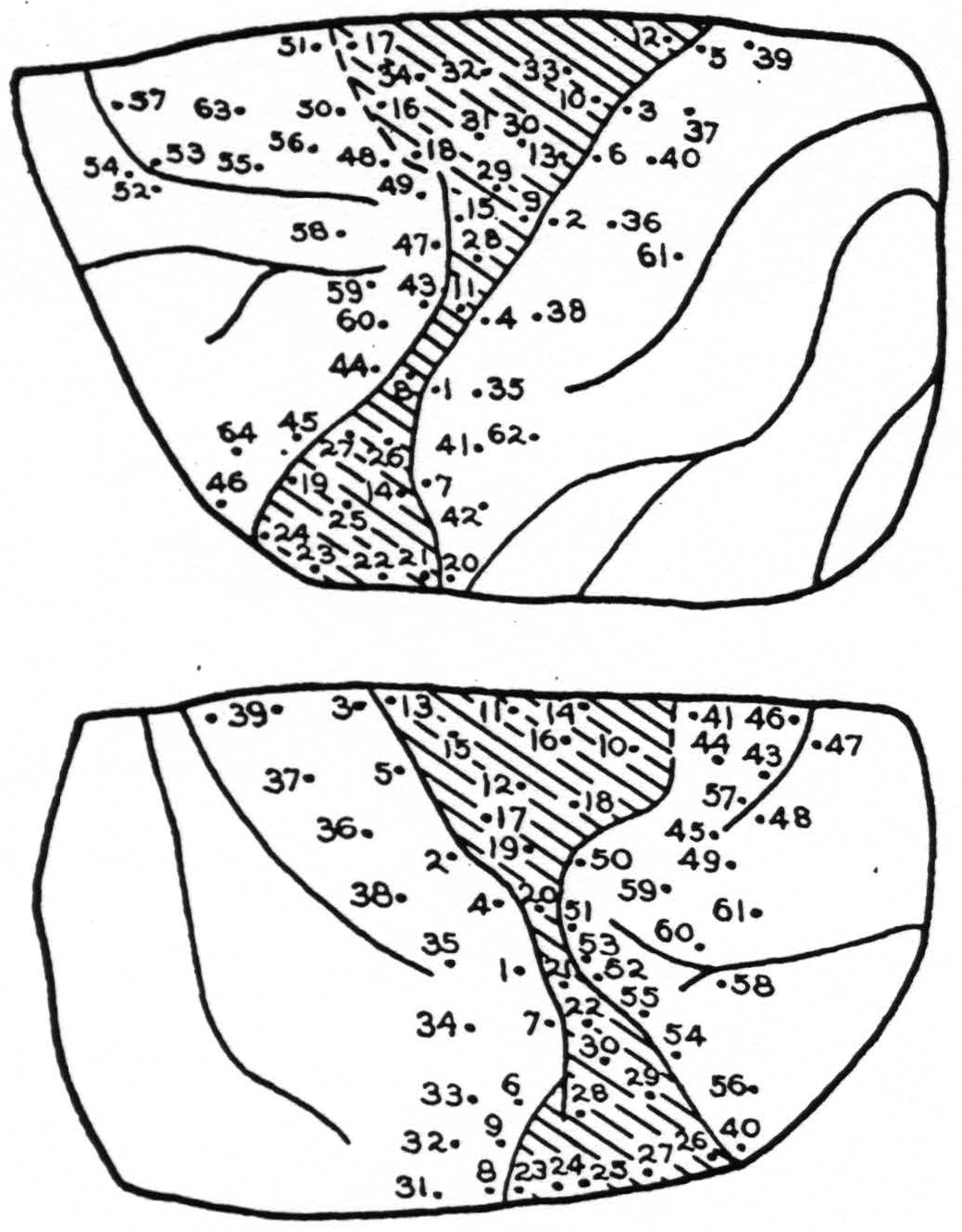
Figure 4.
Summary: Area 8 was intact and gave characteristic responses. Within the scar, movement of wrist and hand were obtained from one area. These were diffuse and irregular. Areas 3 and 1 on the posterior lip of the central sulcus were excitable. All movements were in the arms and face and were diffuse and generalized. In this animal as in the preceding three, the voltage threshold of stimulus producing movement was high, far higher than in the normal cortex, both in the scar tissue and the surrounding regions with the exception of area 8. This latter area normally requires a stimulus of longer pulse form and longer duration than do areas 6 or 4. There was no detectable alteration of the excitability of area 8 in the present instances.
Experiment 5 (I 24). Mar. 16, 1942. Male, weight 3.4 kg. Born Apr. 24, 1938. Ablation of left areas 4, 3, 1 and 2; Dec. 9, 1938; ablation of right areas 4, 3, 1 and 2; cord transection at Th. 5, Feb. 25, 1942.
After the bilateral cortical ablations and preceding the cord transection (which was done for another purpose) this animal had definitely deficient motor performance, although it was not nearly as disabled as those of the preceding three experiments. It was able to use its hands accurately for feeding, although fine movements of prehension were impossible. It ran and jumped awkwardly. Placing and hopping reactions were absent on both sides. It was moderately spastic. To save space the map and list of points are omitted here and the findings summarized below.
Summary: On both hemispheres, responses to stimulation of area 8 were normal, as they had been in the preceding experiments. Caudal to the scar from areas 5 and 7, no response to stimulation was obtained in either hemisphere. Movements of face and tongue were elicited from each side lateral to the scar of area 4. There was evident spread to this region from stimulation of the scar. Stimulation of area 6 produced movements of arms and fingers characteristic of those usually produced from an intact hemisphere. No suppression was produced in what might have been area 4-s. It was therefore assumed that this area had actually been destroyed at operation.
Discussion
The result of cortical stimulation in the above five instances is sufficiently consistent to be considered valid and therefore merit discussion.
Stimulation of adult animal (control): Stimulation of the cortical tissue about the scar in the older animal produced a characteristic and normal response of eyes and lids from the intact area 8, and of the arm from what was possibly a remnant of the rostral part of area 6, but nothing from either the scar tissue or the postcentral gyrus. These findings confirm many chance observations made previously during stimulations of a large number of cortices of adult or nearly adult monkeys after cortical ablations made, usually, just prior to the stimulation, with one exception, namely, that the post-central gyrus is sometimes but not always excitable. In an intact cortex of Macaca mulatta under proper dial anesthesia as were these animals, stimulation of the posterior lip of the central sulcus will usually produce movement as will stimulation much farther caudally in the parietal lobe.
Stimulation of cerebral cortices of animals operated in infancy: Stimulation of these cortices produced much more movement than did that of the older animal. This might be expected and is consistent with the much greater adequacy of motor function of the animals operated on at early age. Movements elicited from all four “infants” had the same characteristics: (i) In all five animals the excitability characteristics of area 8 were alike and were the properties normally found on stimulation of area 8 in an intact cortex. (ii) Stimulation elsewhere always required a stimulus of much greater intensity and often of either longer duration or of longer pulse form than normal. (iii) Responses were always diffuse and poorly localized. There was obvious conduction of stimulus along scar tissue such that a given response (i.e. tongue, Expt. 2) could be produced from a wide area of scar tissue. This spread of stimulus seemed greater than that which is found in any scar tissue and furthermore, the same property appeared in all other excitable areas with the exception of area 8, and, in one instance (Expt. 5), area 6. The spread of excitability did not confirm in time relations or in geographical distribution to that which would be expected from facilitation. It appeared directly related to the diffuseness of response to all stimuli. It may be related also to the lack of discreteness in movements during life. (iv) In every instance the excitable regions were those bordering on the scar and those which, in the intact cortex also, are excitable.
A great number of the movements were of tongue, lip and face and were directly related to the more complex movements produced by stimulation of area 6b. Others appeared on stimulation of the post-central gyrus, but were more diffuse and required higher threshold for response than were elicited from the same region of an intact cortex. In the three animals in which the lower extremities were responsive this obtained in the leg area as well as in arm and face. Movements characteristic of stimulation of area 6 were produced from the animal in which area 6 had not been removed at operation (Expt. 5) and did not differ in any way from those produced from the same area of an intact cortex. In the other animals occasional diffuse arm, neck and shoulder movements occurred associated with movements of the eyes. These likewise can be produced at the junction of areas 6 and 8 in the intact cortex.
Causes of differences: Stimulation of the control cortex and those of the four animals operated in infancy demonstrated marked differences between the two types since much more movement was produced from tissue around the scar in the latter than in the control. This would be attributed to any one of several causes.
(i) The most obvious possibility is that the extirpations of the four animals operated on in infancy were less complete than that of the control. This is unlikely for the following reasons: (a), grossly, at autopsy, the scar and the surrounding tissues were the same in extent on all five animals; (b), examination of the histological sections of all five preparations showed about the same extent of each lesion. In every instance there were large motor, possibly Betz cells, on the posterior lip of the central sulcus, on the inferior lip of the cingulate sulcus and, in a few instances, in the anterior lip of the arcuate sulcus. They were not different in size nor in number from the cells of the same type normally found in these sites in the macaque cortex. There was no other indication of any histological difference between the cortex of the animal operated on late in life and those operated on in infancy; (c), previous findings on a large number of monkeys operated on either in infancy or later have been the same, namely that there is always a great deal better motor function in the animals operated on in infancy than in the adults, and that this is without any demonstrable difference in anatomy or histology.
(ii) The second possibility is that there is a change in the excitability of the remaining tissue normally concerned with motor function. From the present data this seems to be the most probable, for, in the present four cases the tissue surrounding areas 4 and 6, or the scar left by their ablation, was more reactive to electrical stimulation than is that of the intact cortex. However, no excitable region or regions were found in these cortices which would not be excitable in the intact cortex also. The difference was one only of degree. The most marked change in those animals operated on in infancy was the great spread of response from stimulation of one point, and, along with this, the similarity of the response obtained from a number of adjacent or fairly widely separated points.
The above explanation of these phenomena fits both the excitability characteristics of the cortices, and the functional characteristics of motor performance in these animals. It is one that has been offered before and has been described previously in the literature, namely that there is a reorganization of remaining tissue within a partially damaged functional unit (1), that of the motor system in this case. There is, at present, no evidence for anatomical reorganization accompanying this although, in the presence of functional reorganization, some anatomical change permitting wider spread of impulses through synaptic connections is still possible.
Summary
- Stimulation of the cerebral cortices of Macaca mulatta from which areas 4 and 6 had previously been removed has revealed marked differences in excitability of the cortex from which the motor areas has been removed in infancy as compared to that from which motor areas have been removed later in life.
- The cortex of the animal with motor areas removed in infancy has greater excitability in the regions surrounding areas 4 and 6, namely the posterior lip of the central sulcus, areas 6b and 6a than has either the normal macaque cortex or that of the animal from which the motor areas have been removed at a later age.
- Movements elicited from these regions are more diffuse and require a higher threshold stimulus than do these regions in the intact hemisphere.
- No regions other than those known to be excitable in the normal animal were found to be excitable in these preparations.
- The changes in excitability in the animals operated on in infancy are consistent with the well developed motor performance of such animals during life.
- They are consistent with functional reorganization within a partially destroyed motor system. There was no evidence of anatomical reorganization.
Footnotes
References
Kennard, Margaret A. Cortical reorganization of motor function: Studies on series of monkeys of various ages from infancy to maturity. Arch. Neurol. Psychiat., Chicago, 1942, 48: 227-240.
Jacobsen, C. F., Taylor, F. V., and Haslerud, G. M. The effect of cardiac sympathetics and adrenalin upon ventricular rhythms induced in cats by inhalation of petroleum ether. Amer. J. Physiol., 1936, 116: 111-112.
For further research:
Wordcloud: Ablation, Animals, Areas, Arm, Central, Characteristic, Control, Cortex, Cortices, Difference, Diffuse, Elbow, Elicited, Excitable, Experiment, Extension, Eyes, Face, Fingers, Following, Function, Hand, Infancy, Intact, Lids, Lip, Marked, Motor, Movements, Normal, Obtained, Operated, Point, Produced, Regions, Removed, Reorganization, Response, Scar, Shoulder, Spread, Stimulation, Stimulus, Sulcus, Tissue, Tongue, Upper, Wrist
Keywords: Areas, Cortex, Response, Animals, Regions, Monkeys, Function, Experiments, Cortices, Gyrus
Google Books: http://asclinks.live/dguq
Google Scholar: http://asclinks.live/bedp
Jstor: http://asclinks.live/dpsm