PHYSIOLOGICAL NEURONOGRAPHY OF THE CORTICO-STRIATAL CONNECTIONS12 [47]
J.G. Dusser de Barenne, Hugh W. Garol and W.S. McCulloch
Introduction
Several researches by the senior author have established (a) that strychnine locally applied acts strictly locally, (1, 2) (b) that strychnine excites only where there are synapses on nerve cells,(3, 4, 5, 1, 2) (c) that it produces disturbances which travel only in the direction of normal conduction, not antidromically,(4, 1, 2) and finally (d) that these disturbances are diminished, dispersed and delayed when they reach the next synapses(4, 1, 2). From all of which it follows that local strychninization and recording of electrical activity constitute a method for delimiting the axonal distribution of nerve cells in any gray mass of the central nervous system(6), a procedure which we would call “physiological neuronography.”
Electrical records are easily obtained but notoriously difficult to interpret. With intracerebral electrodes the shaft of the electrode must be insulated if the disturbance is to be referred to its tip. This has always been done, and, to prevent any break in the insulation, these electrodes have regularly been encased in a hypodermic needle extending to within 2 mm. of the tip, and thoroughly grounded. But this would not be enough by itself. What we amplify and record are voltages. We are able to do so only by allowing a minute current to flow, which requires a closed circuit. In other words, we must make contact with the animal at two places, i.e., by two electrodes, and the voltage we record will be that appearing between these electrodes. This means that one cannot ignore the position of either electrode. Nor does one escape this difficulty by grounding one of them. Fig. 105 is an actual oscillographic record taken from an artificial monkey made of resistors and capacities to show how the recorded voltages appear with the grounded lead in several places. As the ground recedes from the site of the disturbance the wave forms become
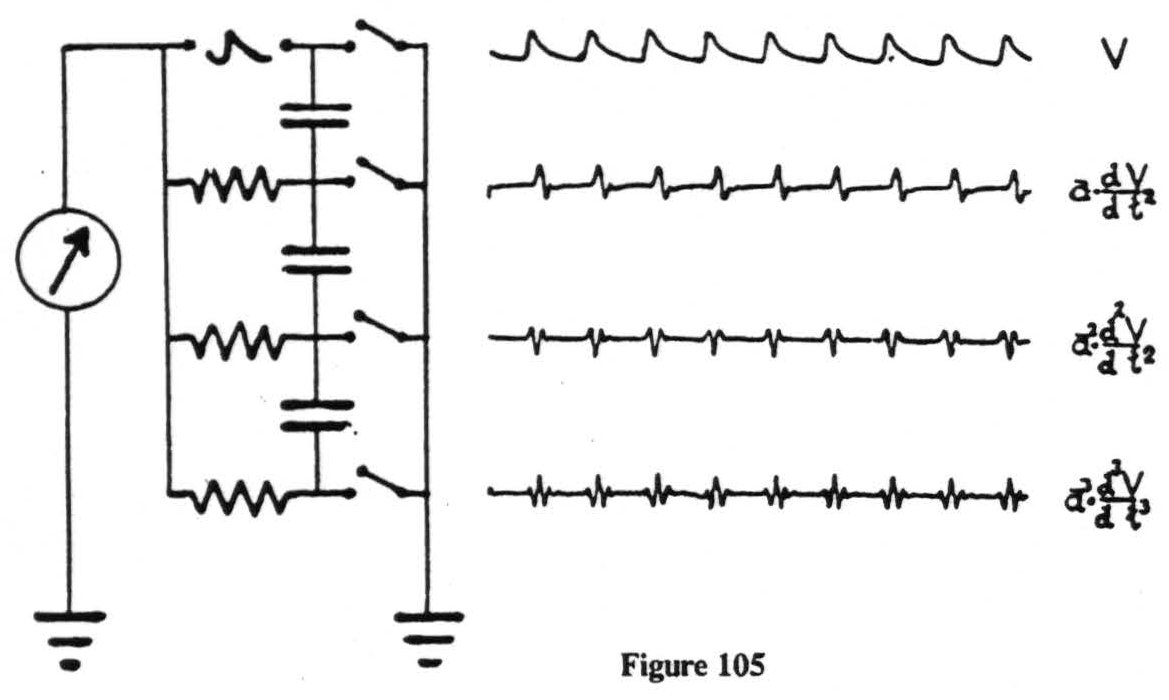 more complicated. Note that the oscillograms presented here are in each case simply fluxions, or derivatives, of the preceding form and mathematically simpler than can be expected from any real monkey. We have therefore usually kept both electrodes ungrounded. Under these circumstances it is obviously impossible to tell which electrode is picking up the disturbance. But if several amplifiers can be simultaneously connected to three or more electrodes, a disturbance can be localized to a single electrode.
more complicated. Note that the oscillograms presented here are in each case simply fluxions, or derivatives, of the preceding form and mathematically simpler than can be expected from any real monkey. We have therefore usually kept both electrodes ungrounded. Under these circumstances it is obviously impossible to tell which electrode is picking up the disturbance. But if several amplifiers can be simultaneously connected to three or more electrodes, a disturbance can be localized to a single electrode.
For example, if one inserts three electrodes as in Fig. 106, and then strychninizes the nucleus gracilis or cuneatus one finds in the records of channels 2 and 3 mirror image records of a strychnine spike. This occurs therefore at the electrode which these amplifiers have in common, i.e., electrode C which is in the globus pallidus and one is therefore entitled to conclude that from the nucleus gracilis or cuneatus run axons to the internal segment of
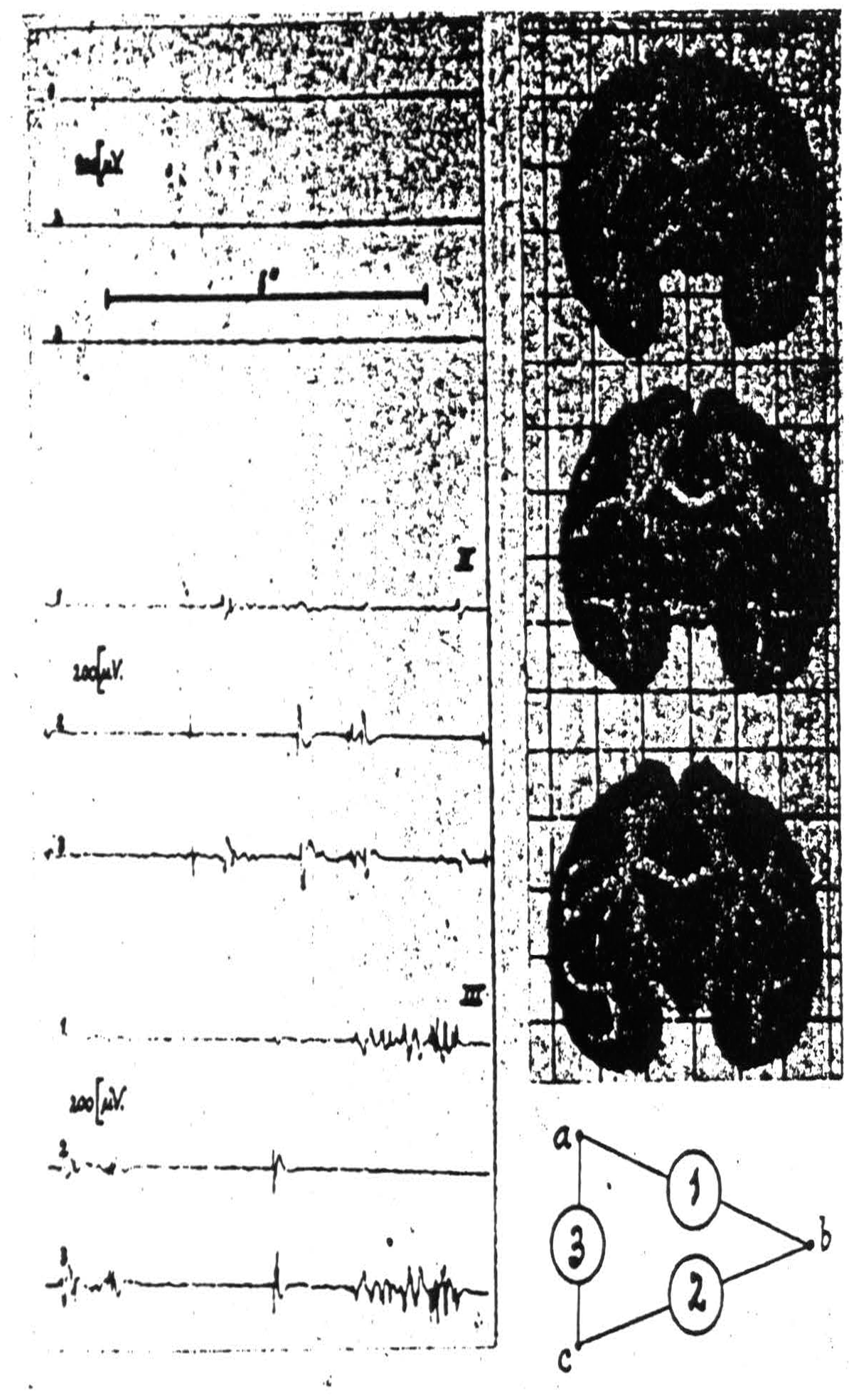
Figure 106. Macaca mulatta. Dial-narcosis. Electrograms taken by the “triangulation” arrangement (see text) from pallidum and putamen. Position of electrodes and their “hook-up” at right of records. Record I (control) before, records II and III after local strychninization of contralateral nuclei of Goll and Burdach. Simultaneous disturbances in electrograms 1 and 3 denote “firing” at electrode a (in pallidum), such in electrograms 2 and 3 firing at electrode c (in pallidum). Absence of synchronous disturbances in electrograms 1 and 2 signifies absence of firing at electrode b (in putamen).
the globus pallidus but not to the putamen, a finding of interest with respect to the basal ganglia and only twice foreshadowed anatomically, by Bechterew's study of myelinogenesis and by Cajal's description of collaterals from the mesial fillet to the globus pallidus.
Fortunately, for the experiments to be presented, it is not necessary to interpret the records beyond the recognition of strychnine spikes and of a general change in the level of spontaneous activity and to assign these to their proper places.
In 1937 we were engaged in mapping by these methods the functional organization of the sensory cortex of the monkey (7) and ran into difficulty with a narrow strip of cortex at the anterior margin of area 4, cf. Fig. 107. When this narrow strip is stimulated electrically, chemically or mechanically, there follows, after a latency of minutes, a transient diminution of electrical activity first seen in area 4 and later observed to sweep across the entire sensory cortex and even beyond it (8). Since minimal strychninization of this strip of cortex — we called it area 4S — need not give any strychnine spikes in the areas showing suppression of their electrical activity it seemed improbable that cortico-cortical connections were responsible for the suppression. To exclude them an incision was made just posterior to area 4S down almost to the nucleus caudatus. The suppression could still be elicited, whereas undercutting 4S prevented it.
As we already knew not only that strychninization of any sensory thalamic nucleus fired the corresponding subdivision of the sensory cortex, but also that strychninization within any subdivision of the sensory cortex fired the corresponding sensory thalamic nucleus,(9, 10, 11) and as we knew that the so-called spontaneous cortical activity remains after decapitation and decerebration but vanishes when the cortex is separated from the thalamus,(12) it was only natural to suspect that suppression of that electrical activity depended upon impulses descending from 4S to the thalamus and there interrupting the interplay of cortex and thalamus responsible for their “spontaneous activity.” We therefore attempted to make a lesion in the thalamus. The suppression obtained before could not be obtained thereafter. We then sliced the brain to locate the lesion and found the thalamus
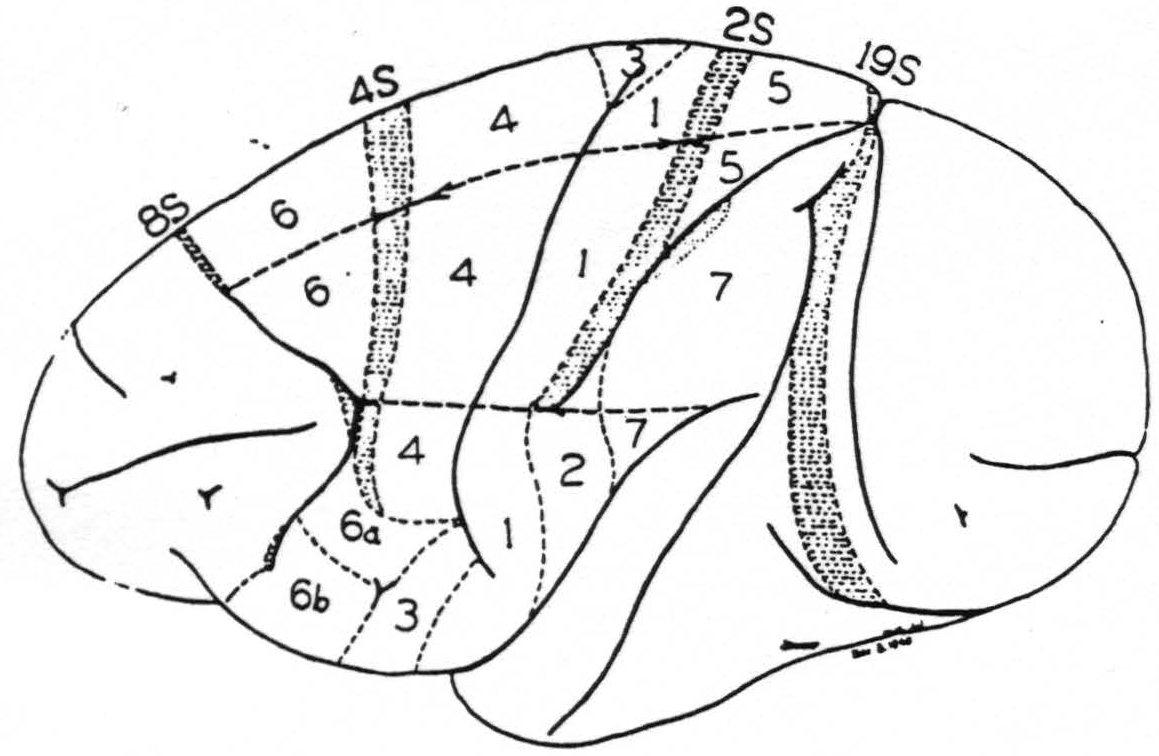
Figure 107. A new map of the sensory motor cortex showing suppressor areas 4S and 2S and indicating the location of the adjacent precentral (8S) and postcentral (19S) suppressor areas. On the sensory cortex is indicated by stippling the area of “visuosensory” firing. This is the only part of the sensory cortex so far known to be fired by strychninization of any cortical area lying without it. Boundaries between leg-, arm- and face-subdivisions are also indicated.
intact. We had pithed the nucleus caudatus. Fearing that, in approaching the nucleus caudatus, we might have cut a tract descending near it to some other structure, we tried it from every direction. The result was the same. Here it should be added that neither a large lesion in the putamen nor one in the globus pallidus, only that in the nucleus caudatus, prevented suppression. We could therefore conclude that the nucleus caudatus is necessary for suppression of electrical activity of the cortex, if that suppression is to be produced by strychninization of 4S.
But this implies, first, that the cortex, area 4S, can affect the nucleus caudatus and, second, that the nucleus caudatus can affect the cortico-thalamic reverberation.
The first of these cortico-striatal relations was checked by placing strychnine on the cortex and recording from the nucleus caudatus. When either area 4 or area 6 is strychninized nothing happens in the nucleus caudatus. Thus, in 4S are situated cell bodies ending in or giving off collaterals to end in the nucleus caudatus. That studies by Marchi's method(13) fail to reveal these endings can only be interpreted to mean that they are not myelinated. It cannot in any way disprove that 4S is connected directly to the nucleus caudatus.
The second of the cortico-striatal relations was checked by strychninizing the nucleus caudatus and recording the electrical activity of area 4. This produced suppression and no strychnine spikes in the cortex. This shows that the nucleus caudatus can effect suppression and must do so by affecting some subcortical structure, for it shows that there are no cells in the nucleus caudatus sending axons to area 4 of the cortex. As a matter of fact, so far as we can discover, it sends no axons to any part of the cortex. That the nucleus caudatus effects suppression of the electrical activity of the cortex by affecting the thalamus can be seen by placing the electrodes in the thalamus, and here the picture is very interesting. Large voltages and blocking of thalamo-cortical reverberations appear, but these voltages lack the characteristic form and are too slow for strychnine spikes. They would therefore incline one to believe that the connection of the nucleus caudatus to the sensory thalamic nuclei is not a simple direct axonal one but has relays somewhere in between.
Thus, while the connection from the cortex to the nucleus caudatus is simple and direct, the connection in the reverse direction is decidedly roundabout, via the thalamus(14).
To forestall false inferences from these facts, let us return to the history of area 4S and suppression. Dr. Marion Hines,(15) working in Baltimore, and we, in New Haven, came upon 4S independently and almost at the same time. She had observed that stimulation of 4S caused sudden cessation of spontaneous activity, and relaxation of existing muscular contractions. We found that stimulation of 4S caused a failure to respond to electrical stimulation of area 4, i.e., a suppression of motor response to cortical stimulation(16) (see Fig. 108). The suppression has a remarkable latency, some 4 minutes, and cannot be produced again for many minutes. It is not a completely generalized suppression of nervous activity. For example, (see Fig. 109) it does

Figure 108. September 28, 1938. Macaca mulatta. Dial. Electrical monopolar stimulation of A. 4 focus once every minute (5"-.6mF.-40/"-700). Extension of wrist. Mechanical stimulation of L. 4-S focus (3) and electrical monopolar stimulation of L. 4-S (4) result in suppression. Time line = 20".
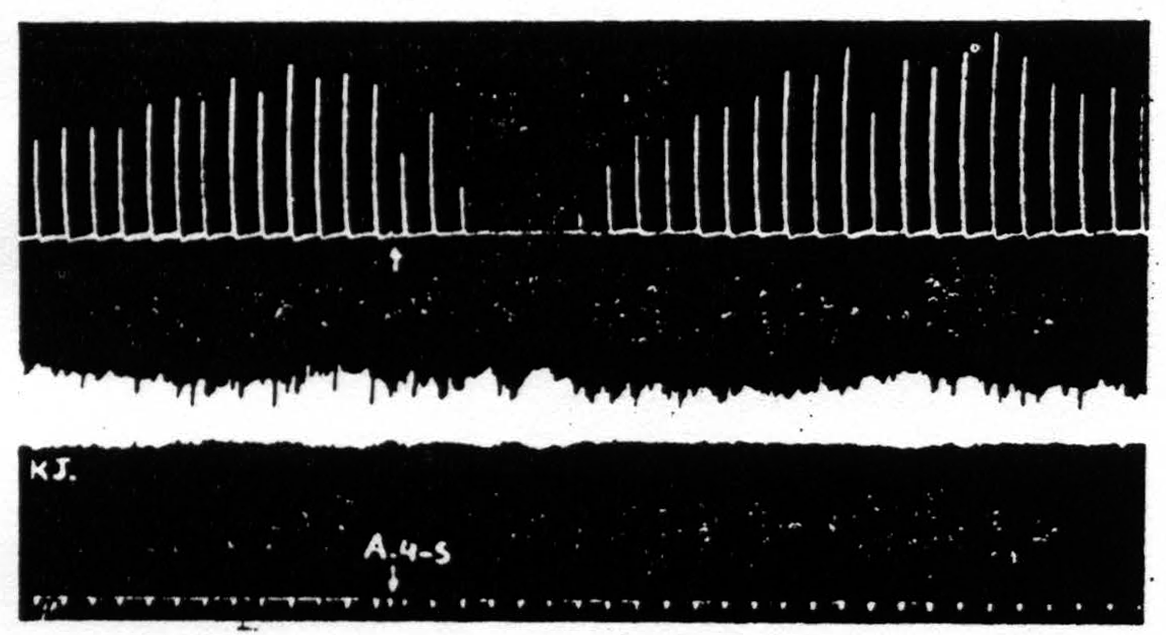
Figure 109. Monopolar stimulation of A4 focus once every minute. Upper record is of extension of wrist, lower record, of knee jerk elicited every 4 seconds by mechanical stimulation of patella tendon. At arrow A4S stimulated resulting in suppression of motor response to cortical stimulation but no suppression of knee jerk.
not affect the knee jerk. So presumably it is restricted to some particular part of the nervous system. It seemed likely that it occurs via the nucleus caudatus, but apparently it does not. Following a lesion which prevented the suppression of electrical activity of area 4 there is still the suppression of motor response. (See Fig. 110.) While we know that for suppression of motor response many structures are severally not necessary (e.g., the nucleus caudatus, the putamen, the globus pallidus, the substantia nigra and the cerebellum) we have no idea how or by what intermediate structures 4S suppresses motor response(16).
What has been said so far is not new but it was necessary to show how one can be certain of the direct path from 4S to the nucleus caudatus and the indirect path back to the cortex, and
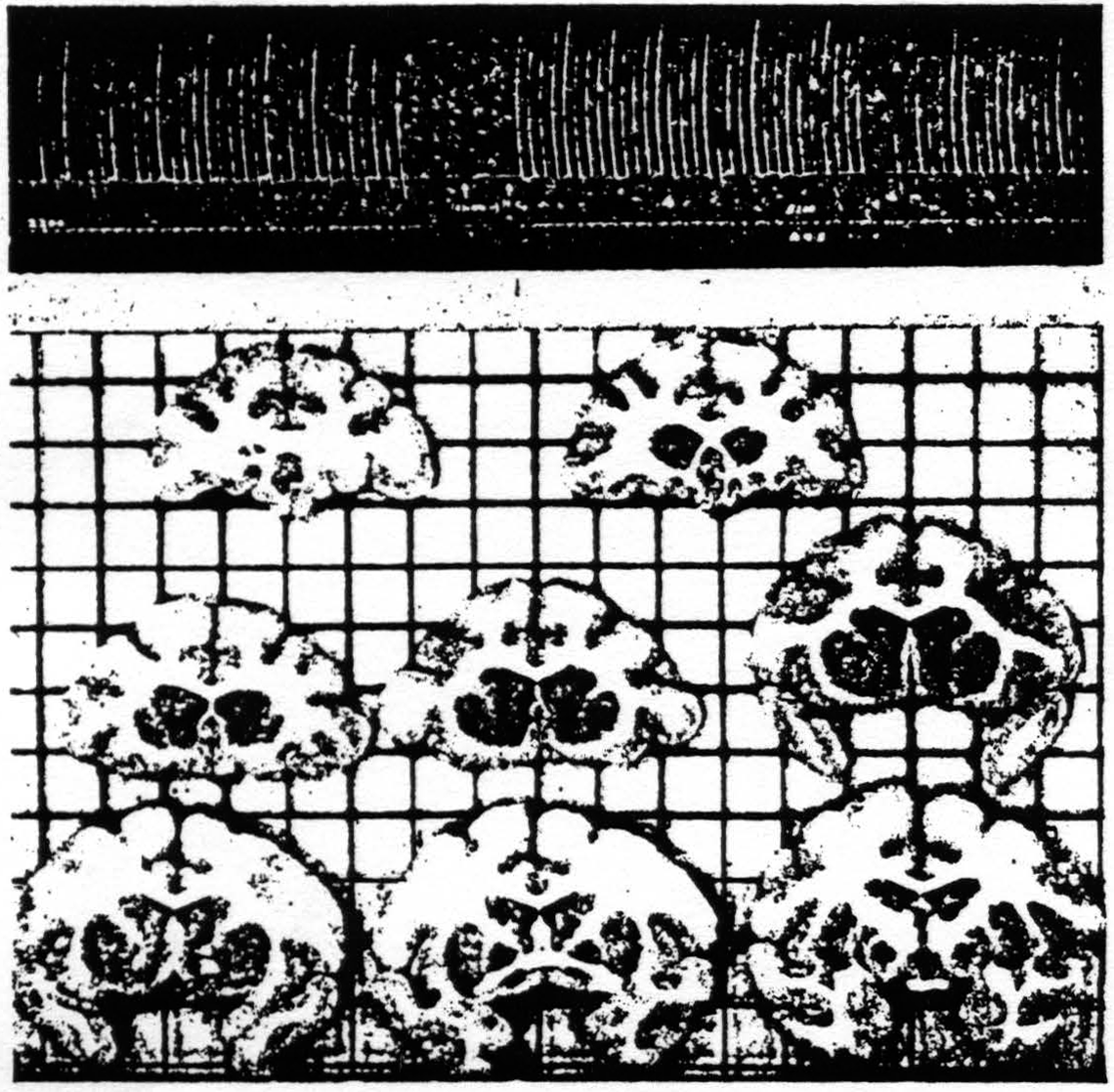
Figure 110. Oct. 21, 1938. Release and suppression of motor response after lesion of nucleus caudatus. Suppression of ECG of A4 prevented by same lesion.
more, to indicate that, while this circuit serves to suppress the electrical activity of the cortex, it is not necessarily involved in suppression of other activity.
These experiments convinced us that it was worth while to make a systematic investigation of the connections of the sensory cortex and the corpus striatum, the results of which we will now consider.
As in the case of the former experiments these new and hitherto unreported ones were performed upon the monkey (Macaca mulatta) under full Dial anesthesia and again consisted of the local application of strychnine and the recording of electrical activity. The difference lay in the use of a greater number of electrodes and amplifiers which enabled the collection of vastly more data on a single animal.
Almost the first of these experiments with multiple electrodes intended for the corpus striatum and several on the cortex, produced new findings. We had obtained suppression of electrical activity of the cortex and firing of the nucleus caudatus by strychninization of the strip 4S lying between area 4 and area 6. We had controlled it posteriorly by strychninizing area 4 which neither suppressed the cortex nor fired the nucleus caudatus, and we intended to control it anteriorly by strychninizing area 6. But the strychnine was placed too far forward, in what we consider to be part of area 8 medial to the sulcus arcuatus because we, like Dr. Marion Hines,(17) have obtained eye movements by stimulating it. It produced suppression of electrical activity of the cortex(8) and it fired the nucleus caudatus. It was then necessary to control it by strychninization still father forward in area 9 which affected neither the cortex nor the intracerebral leads, and by strychninization between 4S and the new suppressor region called 8S, i.e., by strychninization of area 6. This did not suppress the cortex or fire the nucleus caudatus but it did fire an electrode deep in the putamen. (See Fig. 111.) From that time on we went to work with intracerebral electrodes intended for every subdivision of the corpus striatum and with strychninizations of every part of the exposed hemisphere, sometimes 20 of them on a single hemisphere. Fig. 112 shows the type of records obtained. Fig. 113 (sections 4-10) represents the location of the electrodes in the corpus striatum fired by strychninization of the cortex and includes only the 91 positive findings in a total of 280 strychninizations on more than 30 hemispheres. The negative findings would be so numerous as to blur the picture. They would even create a false impression, for it often happens that an electrode in the putamen, for example, is not fired by strychninization of Leg 6 or Face 6 but is by strychninization of Arm 6, whereas another point in the putamen may be fired by Leg 6 and Arm 6 but not by Face 6, and so on. It would have been worth while to record the
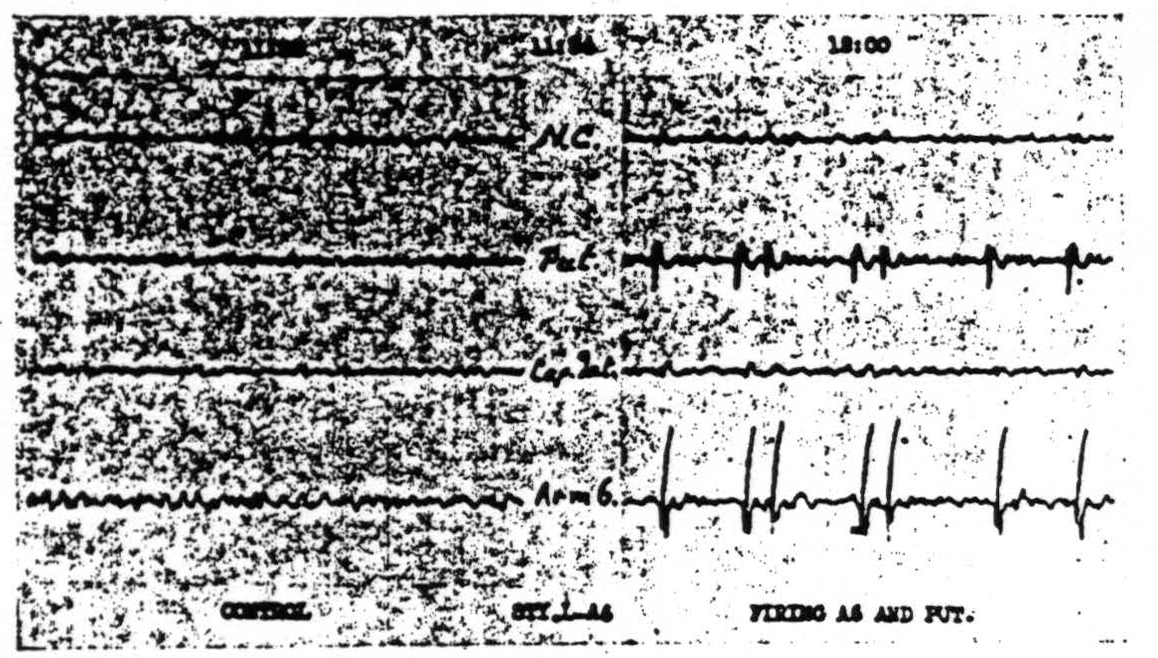
Figure 111. Oct. 11, 1939. Roll II. Strychninization 3. Records of nucleus caudatus, of putamen, of capsula interna adjacent to the lam. med. int. of the globus pallidus and of Arm 6 showing synchronous firing of putamen and of Arm 6 by strychninization of Leg and Arm 6.
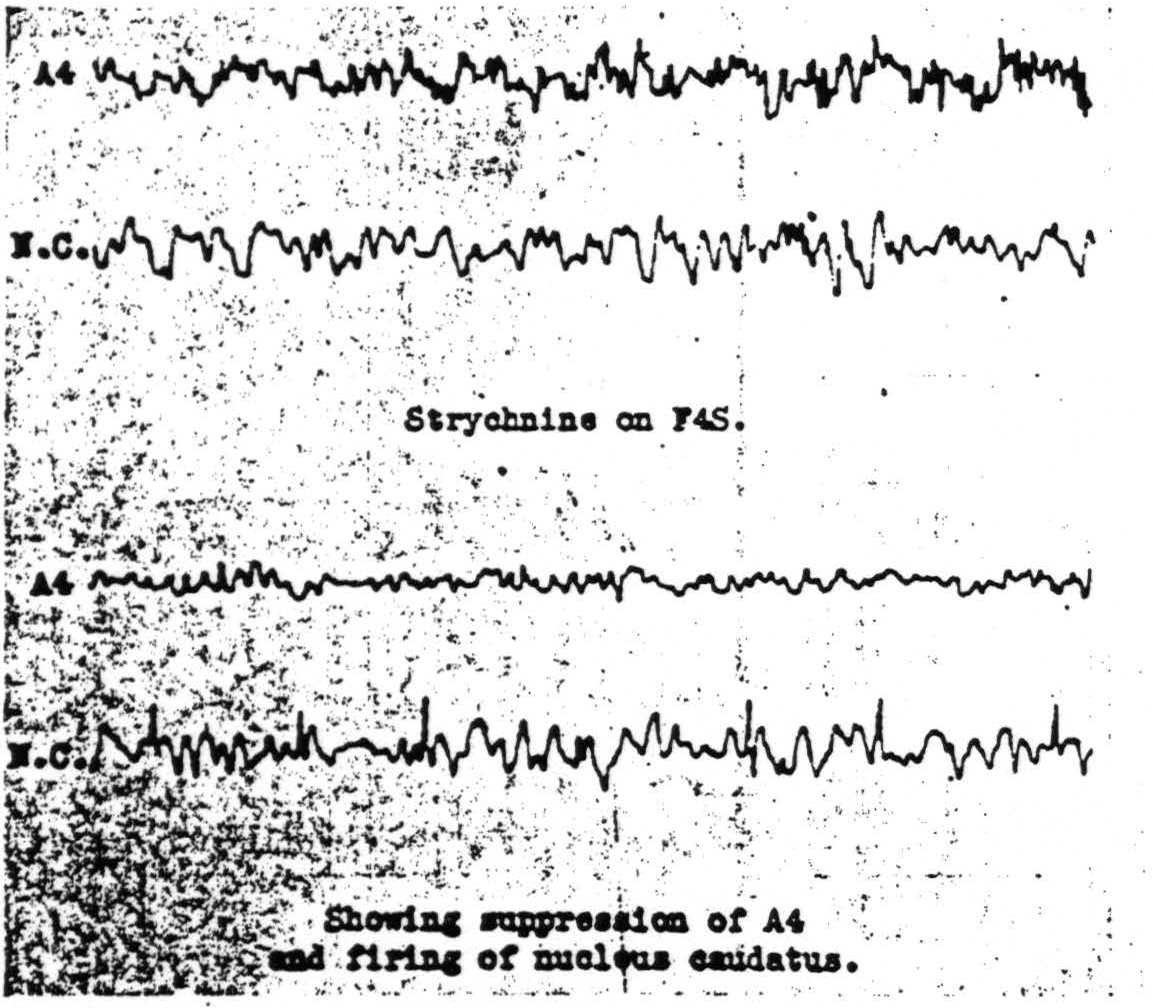
Figure 112. Jan. 29, 1940. Roll VIII. Strychninization 9. Records of the electrical activity of the cortical area Arm 4 and of the nucleus caudatus before and after strychninization of Face 4S of the cortex showing suppression of the electrical activity of the cortex, Arm 4, and firing of the nucleus caudatus.
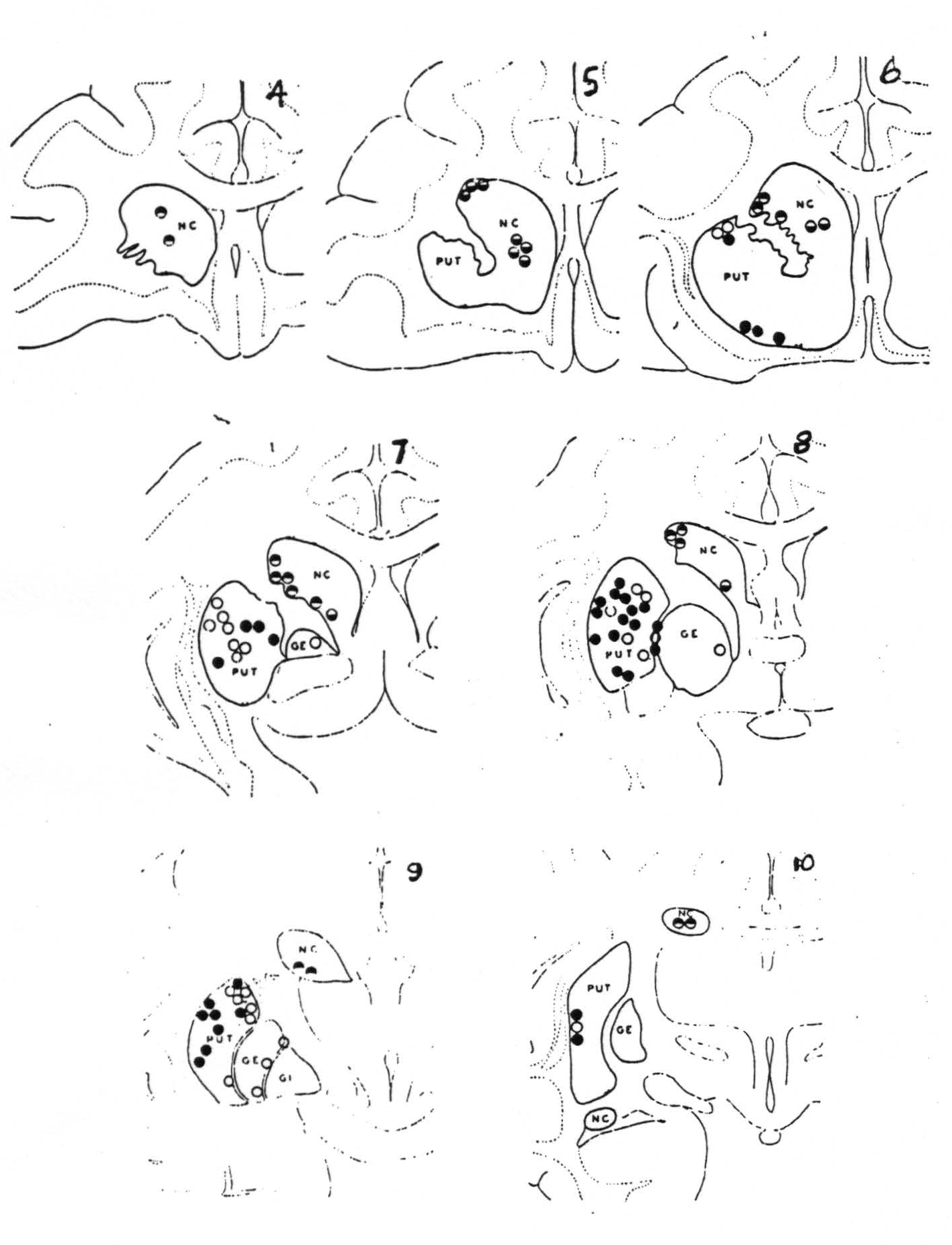
Figure 113. Schematic representation of the location of electrodes as disclosed by sectioning the brain serially into slices from about 2 mm. thick, and numbered from before backward. Black circles indicate firing at these points from strychninization of the cortical area 4 (motor cortex) and white circles, firing from area 6 (premotor cortex). Divided circles indicate firing from strychninization of suppressor bands, black above, from 4S and black below, from 8S.
negative as well as the positive findings had these been such as to demonstrate somatotopic localization in the cortico-striatal projections. The evidence will not do that, for while there is unquestionably firing at any point from one of two rather than from all somatotopic (face, arm, leg) subdivisions of the sensory cortex, the points in the corpus striatum to which these project (L, A, F of Fig. 114) are so intermingled as to defy attempts to plot the somatotopic localization which their existence strongly suggests. If any such localization exists at all it cannot be simple.
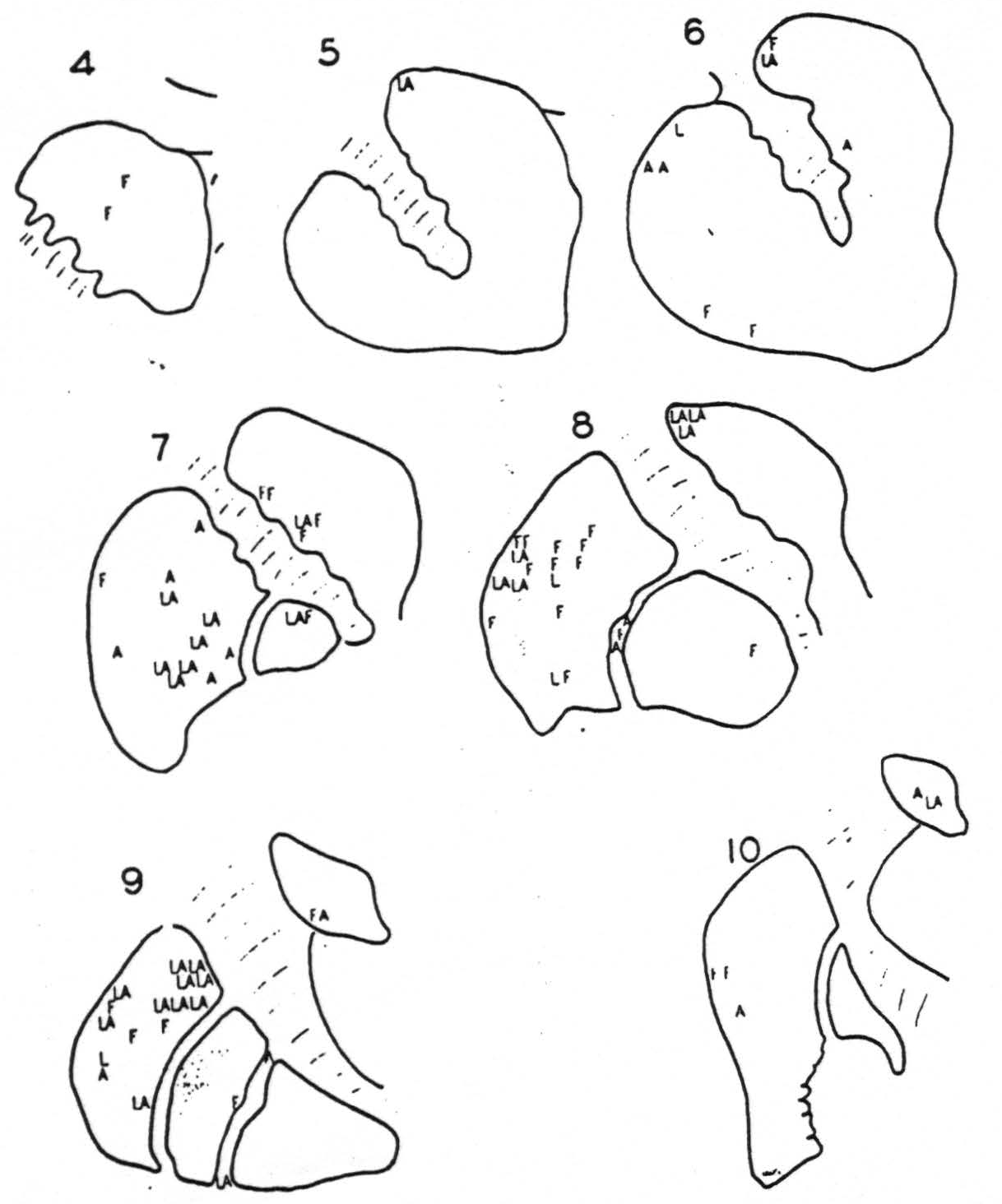
Figure 114. Schematic representation of the corpus striatum on which are letters, L, A and F, representing the locations of electrodes fired by strychninization of the somatotopic subdivision of the cortex. Leg, Arm or Face, to which the letter corresponds. Note the lack of somatotopic localization in the striatal terminations of the cortico-striatal projections.
The general statement of negative findings must include first, that strychninization of no part of the corpus striatum has ever fired any part of the cortex from area 9 to area 19 inclusive. Second, that strychninization of no part of the cortex has ever fired the inner segment of the globus pallidus (that is the part fired by strychninization of nuclei gracilis et cuneatus). Last, that, with one notable exception just discovered, no cortical area lying behind the sulcus centralis has ever fired any part of the corpus striatum. All three negative findings have been repeated systematically. This allows us to concentrate our entire attention on the area of cortex extending forward from the sulcus centralis to just in front of the sulcus arcuatus — an area consisting of 4 bands which are physiologically distinct. (See Fig. 115.)
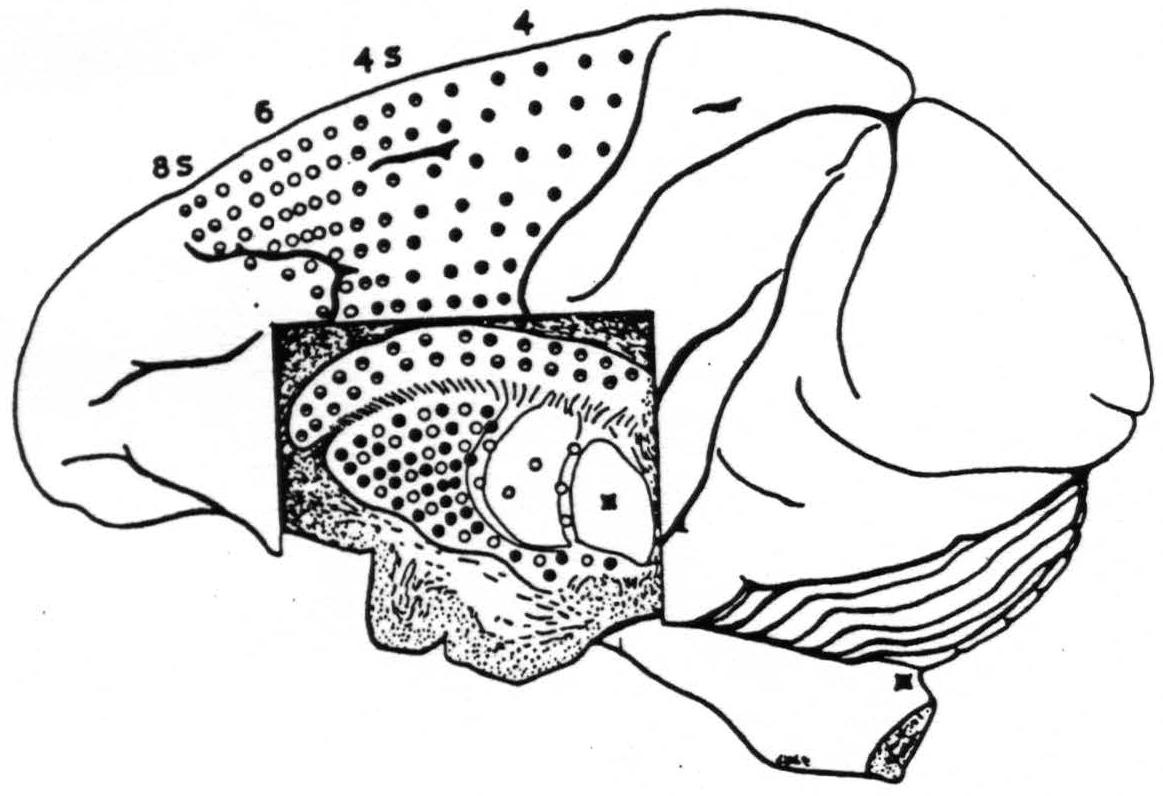
Figure 115. Schematic synopsis of the findings indicated in Fig. 113 upon which has also been indicated the firing of the internal segment by strychninization of N. gracilis and N. cuneatus. Each circle in the basal ganglia corresponds to one experiment showing firing there upon strychninization of a similarly marked cortical area.
Of these the most posterior is 4, the area giganto-pyramidalis, commonly called the motor cortex. Strychninization here fires the putamen but not the globus pallidus and not the nucleus caudatus. For reasons already stated we concluded that this cortical area 4, the “motor” cortex, sends axons or collaterals to the putamen but not to the nucleus caudatus and not to the globus pallidus.
The second, area 4S, sends axones or collaterals to the nucleus caudatus, not to the putamen, not to the globus pallidus.
Next comes area 6, and this again sends axones to the putamen and to the external segment of the globus pallidus but not to the nucleus caudatus.
Last comes area 8S which, like area 4S, sends axones to the nucleus caudatus and not to the putamen, not to the globus pallidus.
Thus, whether or not there be any discoverable somatotopic localization in the corpus striatum, there is, to coin a word for it, a cortico-topic localization and a complex one at that, with only area 6 projected upon the external segment of the globus pallidus, areas 4 and 6 projected upon the putamen and areas 4S and 8S upon the nucleus caudatus. This localization of 8S, 6, 4S and 4 in the cortex, being antero-posterior, is at right angles to the somoto-topic localization there of leg, arm and face, which is dorso-ventral.
From a purely anatomical standpoint, that spatially-separated areas 4 and 6 should project to one structure, the putamen, while 4S and 8S project to another, the nucleus caudatus, is truly surprising, for 4S lies between 4 and 6, and 6 lies between 4S and 8S. On the other hand, when one realizes that 4 and 6 are alike in giving motor responses and that 4S and 8S are alike in giving suppression it becomes highly probable that the projections from 4 and 6 may converge somewhere and that those from 4S and 8S may converge somewhere else, and it ceases to be so surprising to discover these discrete convergences in the corpus striatum.
Fig. 116 schematizes the connections of 8S to nucleus caudatus, of 6 to putamen and globus pallidus externus, of 4S to nucleus caudatus, and of 4 to putamen.
Even while it was being drawn Drs. Garol and Bailey found the notable exception mentioned above. They had an electrode in the posterior superior part of the nucleus caudatus where it is
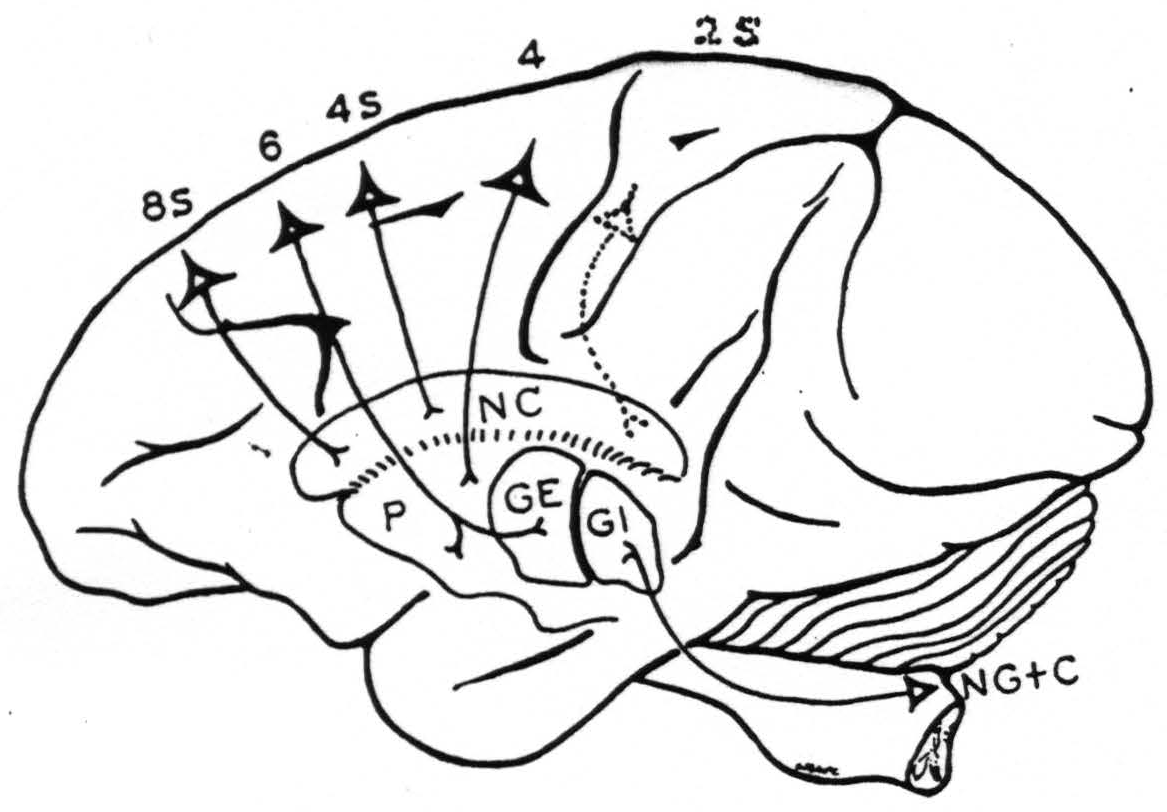
Figure 116. Diagrammatic representation of the conclusions with respect to cortico-striatal connections with a dotted indication to include the findings of Nov. 14, 1940, cf. Fig. 117.
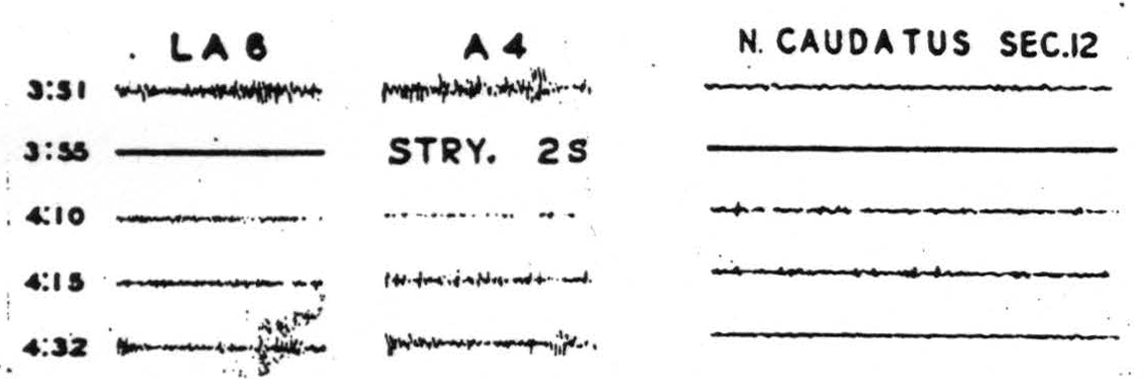
Figure 117. Nov. 14, 1940. Electrical records of the activity of Leg and Arm 6, Arm 4 and of the posterior superior part of the nucleus caudatus before and after strychninization of the post-central suppressor area, 2S, showing some suppression of LA6, more marked of A4 with firing of the nucleus caudatus.
minute and well behind any position from which we had previously recorded. They strychninized the postcentral suppressor strip, suppressed the cortex and fired the nucleus caudatus. (See Fig. 117.)
The schema (Fig. 116) was therefore corrected to include this result. It becomes clearer that cortico-striatal projections of the suppressor regions must be sought in the nucleus caudatus and it begins to look as if there might be some cortico-topic localization within the nucleus caudatus.
Discussion
Dr. Paul C. Bucy (Chicago, III.): No one is sorrier than I that Dr. Bard is not able to be here. I find these not only a most interesting but also a most complex group of papers which it is not easy to discuss.
These experiments of Dr. McCulloch which are extremely interesting and important and which it seems to me have a great deal of bearing upon clinical and pathological observations, begin to draw together in a way that has not previously been possible a great many widely separated observations. Many people from pathological and clinical-pathological studies have, of course, felt that damage to the caudate nucleus was responsible for release of some motor mechanism to produce involuntary movements. There has been a great deal of disagreement as to what motor mechanism was productive of those involuntary movements and we were, of course, happy to have Dr. Fulton from a purely physiological standpoint come to the support of those of us who have followed Kinnier Wilson in believing that fibers arising from the precentral region constitute the motor system which produces involuntary movements. This work of Dr. McCulloch's ties in with that very nicely and supports those who believe that the caudate nucleus when damaged allows this cortical motor mechanism to produce involuntary movements. The fact that Dr. McCulloch believes these impulses to be effective upon the cortical system by way of the thalamus brings agreement with those people, including Dr. Kinnier Wilson and Dr. Paul Schuster, who felt that some of the lesions which released the cortical motor mechanism to produce involuntary movements lay within the thalamus. We all know that there are other mechanism, such as the cerebello-rubro-thalamic, which are capable of inhibiting this cortical mechanism and which when destroyed releases it to abnormal activity. It seems likely that if the findings not unlike those which Dr. McCulloch has been able to demonstrate for these suppressor strips in areas 8 and 6 and now in the postcentral gyrus and the caudate nucleus.
Also, as we can see here a very close correlation between Dr. McCulloch's observations that the caudate nucleus is able to suppress the activity of the motor cortex with that work which Dr. Mettler reported in which he stimulated the caudate nucleus by an entirely different method, an electrical stimulation, and yet produced from a physiological standpoint a similar result.
In connection with Dr. Mettler's observations, I should like to ask him one or two questions. Obviously, Dr. Mettler must have had anatomical controls which, due to the shortness of the period which was at his disposal, he was unable to present to us. However, I should like to ask whether or not from those anatomical controls there was any difference in the results obtained from stimulation of various parts of the caudate nucleus and in the suppression of either spontaneous movements or movements produced by electrical stimulation of the motor cortex. I should like also to pass on a question which came to me from the audience. How as the caudate nucleus narcotized in Dr. Mettler's experiments?
I feel certain that in connection with Dr. Fulton's experiments of destruction of the globus pallidus and other parts of the basal ganglia, we should like to know what Dr. Ranson saw in his experiments in which lesions were also produced in this region.
Dr. Rioch's observations are likewise of great interest to us. I must admit I was not asked to make this discussion until after Dr. Rioch's presentation and I do not have detailed notes on what he had to say. There is one point, however, in connection with his presentation which I should like to make. These are an extremely interesting series of experiments having to do with the physiology of the manifestations, if you like, of emotions in the cat. Obviously we must be very careful and very hesitant in transferring conclusions or facts drawn from these experiments to primates. In the cat we have an animal which with these extensive lesions, including a complete destruction of the cerebral cortex and often a considerable destruction of the basal ganglia, it is still able to spit and claw and turn its head and even walk about and eat its food. Obviously such does not obtain in primates, as Dr. Fulton and Dr. Bieber, Dr. Marion Hines and other people have demonstrated. Whether or not the emotional picture in primates would be comparable to that seen in cats, whereas the motor picture is not, we are as yet unable to say.
Dr. Fred A. Mettler (Augusta, Ga): I should like to ask Dr. Rioch if he made any specific observations with regard to “following” in his animals. Also, there is another phenomenon which is difficult to visualize and that is the “leaping reaction” which some of these animals display.
With regard to Dr. McCulloch's paper, since no doubt it will come up sooner or later and we might as well get the fat in the fire at the beginning, I should like to ask him how he explains the fact that clinicians find a very high degree of localization in the various extrapyramidal diseases which they describe. In conjunction with that, I might observe that we too have made a great many attempts to check the possibility of somatotopical localization within the caudate nucleus, and we have to agree with Dr. McCulloch. We are not necessarily saying that this contravenes anything which the clinician has advanced, but we must agree that we have no evidence that any particular type of localization exists.
Dr. F. H. Lewy (Philadelphia, Pa): I should like to ask Dr. Fulton how his monkey with bilateral destruction of the globus pallidus behaved when sitting on a bar, whether he swayed forward and backward without falling, as I have seen in these monkeys.
Dr. Stephen W. Ranson (Chicago, III.): I would like to ask Dr. Fulton if he can clear up a little more for us the relation of the tremor, which he saw in his animals, to the tremor of paralysis agitans. He stated that the tremor in these monkeys was a tremor of action and it occurred when movement was under way, while, as I remember it, the tremor of paralysis agitans is a tremor of rest which tends to disappear during movement.
Dr. Bucy asked about the symptoms in our monkeys with lesions in the corpus striatum. In the caudate and the lentiform nuclei, we had only unilateral lesions and we saw no symptoms. In the globus pallidus we had bilateral lesions of good size, large bilateral lesions, and in those animals we saw no symptoms other than slight impairment of movement which we were inclined to attribute to secondary involvement of the pyramidal tract. There was in these monkeys certainly no tremor, no athetosis and nothing like a typical masked face; nor was there any considerable slowing of movement. Masked face and slowing of movement we saw very clearly in our animals with hypothalamic lesions but not in these animals with pallidal lesions.
Dr. David McK. Rioch (St. Louis, Mo): Mr. President, the application of the concept of “levels” to the relation of the striatum to the cortex seems to me to be of questionable value. Both the fact that after removal of the neocortex no definite striatal function has been demonstrated, and also the phylogenetic and ontogenetic relationships of the striatum and cortex suggest that these structures are two parts of a functional unit, the cerebral hemisphere.
Dr. Fulton and Dr. Bucy referred to Wilson's hypothesis that involuntary movement were initiated in the cerebral cortex and were released by lesions of the basal ganglia. It seems likely that the involuntary movements of chorea are dependent on cortical activity. Movements described as “athetoid,” however, were observed in anencephalic monsters by Dr. Grinker of Chicago and by two French authors, Nayrae and Patoir. It follows that certain of the involuntary movements included in the composite group clinically designated as athetoid are initiated in sub-cortical, probably in sub-striatal centers.
With regard to tremor and the parkinsonian syndrome, an observation of Delmas-Marsalet and van Bognert is of interest. As an attempted therapeutic measure two lesions were made surgically in the cerebellum of a patient with parkinsonism. This resulted in decrease in the rigidity, but also in marked increase in the severity of the tremor.
Dr. S. Philip Goodhart: I should like to ask Dr. Fulton whether he found unilateral lesions of the pallidus alone produced tremor or whether his observations warrant the conclusion that the pathology must be unilateral.
I should like to know also whether he stated that ablation or extensive injury to area 4 or 6, either of them, was followed by flaccidity. I understood him to say that in lesions with complete removal or extensive ablation of area 6, there followed increased resistance to passive motion. In using the phrase, increased resistance to passive motion, does he mean to say this was the equivalent of rigidity; if this modification of tone was a form of rigidity, was this a parkinsonian type in its distribution, affecting particularly the flexor groups.
Dr. Paul F.A. Hoefer (New York, N.Y.): I would like to ask Dr. Fulton if he wants to classify tremors and athetosis as dysrhythmias, because this seems to make use of a term which has been coined for some other purpose and it also seems to imply that rhythm is normally present and that parkinsonian or cerebellar tremor or athetosis are disturbances of a “normal rhythm.”
I would also like to ask him whether he wants to distinguish physiologically between athetoid and regular alternating dyskinesias. We have reason to believe they are produced by involvement of different systems and presumably also transmitted by definite pathways. Kinnier Wilson did not have a clear attitude as to the function of the pyramidal tract in those conditions because in one place he stated that things are transmitted by it and that it should be “relatively” intact. In other parts of his writings, he has stated that certain dysfunctions of the pyramidal tract are necessary to produce some of the dyskinesias.
As to Dr. Fulton's remark that the distinction between voluntary and involuntary movements become less sharp, I heartily agree with that. It is, of course, very difficult in an experimental animal to know what is voluntary and what is involuntary, but even in man this distinction becomes less sharp, as for both voluntary and involuntary movements apparently at least two different motor systems can be utilized, which both also are utilized in dyskinesias.
Dr. H. Garol (New Haven, Conn.): I would like to ask Dr. Mettler (it is an enlargement on a question that has already been asked) how he can narcotize the nucleus caudatus to the exclusion of anything in the immediate proximity and really get all of the nucleus caudatus.
The second question I have to ask has a strong bearing on the suppression of motor response or inhibition. Is stimulation of the contralateral nucleus caudatus simultaneous with stimulation of area 4? Will you get suppression or inhibition of activity? Is there a bilaterality of that during electrical stimulation of the motor cortex?
President Putnam: I am sorry to say if we are to maintain the record, we must be brief with the discussion from this point. I think I shall close the questions, merely making one observation, if I may do so, in regard to parkinsonian tremor. I think there are many clinicians who would disagree with both Dr. Fulton and Dr. Rioch in regard to the nature of parkinsonian tremor, and I believe Dr. Parkinson would disagree with it. (Laughter) To be sure, we are usually left with the impression that to be the real parkinsonian, a tremor must be a tremor of rest, and many men since, I think, Celsus, have emphasized the fact that tremors could be divided into two types. I believe, however, that it has been the experience of almost everyone who has had to do with any patients suffering from parkinsonian syndrome, either the senile type or the post-encephalitic type, that in some instances the tremor appears only at rest; in other instances, it appears practically only in motion, and in some instances it appears in both. I think we must look in other directions, especially in the direction of the distribution of impulses and the pattern of motor unit innervation for differentiation between alternating tremor and athetosis which I personally feel can be made very distinctly in typical instances.
Dr. David McK. Rioch (St. Louis, Mo.): Dr. Bucy asked about the application of data obtained from experiments on carnivores to primates and man. No dogmatic statement can be made in answer. Observations have been made on anencephalic humans with practically no functional tissue in front of the trigeminal nucleus. They have been seen to turn their heads to a sound; spit out quinine, but to suckle on milk; to cry when they are pinched. Anencephalic monsters in which the hypothalamus is also intact cry excessively when they are not fed and stop crying when they are fed. They also sleep and show mimetic movements of the face. Most observers have been rather impressed with their surprisingly close resemblance to normal babies.
I think on the basis of these observations one is justified in applying the data from carnivores to higher forms. The encephalization that apparently occurs in higher forms is not so much on the efferent side as it is on the afferent and analytical. It results in ability to deal with more complex situations, especially with temporally complex situations. There is neither evidence that phylogenetically the extrapyramidal systems become smaller or less differentiated nor that they lose the functions that they had earlier. The evidence is more strongly in favor of the hypothesis that new and more complex functions are added by the cortex.
Dr. Fred A. Mettler (August, Ga.): With regard to the question concerning anatomical controls on these particular experiments, the diagram which I will present in just a moment (I have a slide for it) represents 200 individual placements made in the caudate and in accessory structures.
(Slide) In this particular slide, all of which, unfortunately, doesn't show here, the areas which are marked by horizontal lines show the particular points at which inhibition of spontaneous movement alone was elicited, whereas the points which are marked with vertical lines indicate the region at which cortically induced activity could be inhibited.
Now, there are a number of apparent reasons why this situation is as it is. In the first place, as one draws near (it would be more apparent if we could see the first two sections of the slide) to the subcallosal fasciculus, what seems to be happening is that one is stimulating a bundle running into the caudate. Whereas, when you stimulate in other portions, more outlying portions, it is very difficult to activate more than a very restricted part of the caudate. Now, if the internal capsule is previously degenerated out, a more extensive region gives inhibition than this particular slide would indicate. So that the inhibition which is produced is not the result of stimulating the capsule, quite the contrary. Stimulating the capsule often produced movements and obscures what inhibition you might get.
The question concerning narcotization of the caudate is a very good one because it brings out a number of important points. One of them is this: it is, of course, impossible to narcotize structures such as the caudate nucleus exclusively and completely, and if we introduce a large amount of cocaine or novocaine or something of that sort through a syringe in the Horsley-Clark instrument, one would assume that what would happen would be that you would get some narcotization of fibers of passage. Well, if we narcotize, let us say the region of the caudate in an animal in which the pyramids are intact, what happens is that you get an increase in spontaneous activity and if you stimulate the cortex under such circumstances, you don't see the same degree of inhibition that you would see if the structure were not narcotized. So that I think it can be said that although it is certain that the anesthetic mixture will diffuse beyond the caudate, the effect which is obtained is not the result of narcotization of fibers of passage.
Dr. John F. Fulton (New Haven, Conn.): Mr. President, since the time is getting on, may I make a general comment first about the tremors? We have attempted to show that the tremor just described is essentially a tremor of action, tremor associated with volitional movement, tremor that was not seen during states of complete relaxation. For this reason it is quite unlike the usual parkinsonian tremor, but in keeping with Dr. Putnam, I have always been a little skeptical of the hard-and-fast distinction between tremors of rest and tremors of action. The only persistent tremor of rest that I have ever seen experimentally in an animal has resulted from a cerebellar lesion. Ablation of the cerebellum, as many of you know gives rise to persistent rhythmic movements that are seen when the animal is sitting quietly in the corner of its cage. We have never seen anything of that character as a result of lesions of the basal ganglia or combined lesions of the basal ganglion cortex, and our attempt has been to describe the phenomenon as clearly as possible and see whether in the course of time it may correlate with anything that has been seen in clinical cases. At present we make no claim for a correspondence between these tremors which are so conspicuous and so striking in these animals and corresponding clinical syndromes.
I think that perhaps will answer the point raised by Dr. Rioch and Dr. Ranson and to a certain extent the point of Dr. Hoefer. Time would preclude a detailed discussion of the possible morphological basis of the choreas and athetoses. However, as mentioned in the paper, Dr. Kennard in one of her baby monkeys saw a most characteristic instance of athetoid movements following a basal ganglion lesion. Again, we have had the striking example in a chimpanzee of a choreiform movement that continued in a conspicuous manner for two weeks, then largely subsided. We as yet do not have anatomical data which would allow any generalization concerning the anatomical correlations that we may possi-bly, and we hope we can make later with these clinical symptoms.
In Dr. Bucy's discussion, he mentioned that we agree with Kinnier Wilson that the corticospinal system's integrity was essential for the appearance of these tremors. That wasn't exactly the point. If area 4 is removed, these tremors may still occur. Our results indicate that some motor projections, be they extrapyramid or pyramidal from the precentral convolution, must remain in part in order for the manifestations to appear.
Dr. Lewy asked whether we had ever put a globus pallidus monkey on a bar. I think I will refer that question to Dr. Kennard. I never happened to see one on a bar. Their equilibrium is certainly not too good.
The question by Dr. Ranson about mask-like face, I am afraid we can't answer, because in order to destroy the globus pallidus it has been essential to destroy the face areas, or at least considerably to interfere with them, and these animals have a very conspicuously mask-like face, but that is secondary, possibly, to the interruption of the face projection. It is true, however, animals with area 6 and caudate out are less grimacing creatures than normal monkeys.
In response to Dr. Rioch's question about levels of function, I am not quite sure that we would follow his argument, namely, that since the cerebral cortex is developed pari passu with the basal ganglia, the cerebral cortex may not be dominant over the striatal regions. The neocerebellum has developed pari passu with the cerebral cortex, yet I don't think Dr. Rioch would be inclined to refer to the neocerebellum as the chief hierarchy in the Hughlings Jackson sense. There is evidence of an increasing dominance of the cortex over all phases of motor adjustments and it is my personal belief — I am not sure whether Dr. Kennard would agree with me in this regard — that the dominance is as manifest in the cortico-striatal relations as it is in the relations with the lower motor centers.
In view of the time, I cannot discuss Dr. Goodhart's question fully. It is true, as he said, the ablation of area 6 gives paresis associated with increased resistance to manipulation and that area 4 ablations in the first weeks following the ablation are essentially flaccid.
Dr. Warren S. McCulloch (New Haven, Conn.): First, I would like to thank Dr. Bucy for his sympathy in my trying to keep up the good record. What I had intended to say was that the area that we searched carefully was the area which extended from a point just in front of the sulcus arcuatus to the sulcus centralis, and I probably ran the two statements together.
The next point was a question by Dr. Mettler. He asked me how I could explain the lack of somatotopic localizations within the corpus striatum which certainly are experienced clinically. Now, first, as to the fact. Our experiments are on the cortico-striatal projection and nothing that we have said or have found could show one way or the other, whether or not there were somatotopic localizations in the corpus striatum due to its projection to lower systems. It might well have such an effect, but I would be grateful to any of you, particularly to any clinicians who will take the trouble to look at the posted diagrams showing the actual distribution of face, arm and leg pick-up in the corpus striatum upon strychninizations of the cortex. If you can make any sense of it, I would be grateful.
Finally, in answer to Dr. Ranson, being myself primarily not an anatomist, I am rather chagrined that having discovered the projection from the nucleus gracilis and cuneatus to the internal segment of the globus pallidus, we found that the anatomists were ahead of us. Cajal had stated there were collaterals giving off from the mesial fillet on its way past, and, by myelinogenetic studies, Bechterew had found the tract before him.
Footnotes
References
Dusser de Barenne, J.G. Die Strychninwirkung auf das Zentralnervensystem. Die segmenture, streng lokalisierte Strychninvergiftung der dorsalen Rilckenmarksmechanismen: ein Beitrag zur Dermatomerie der hinteren Extremitut des Hundes. Zbl. Physiol., 1911, 24: 1100-1102.
Dusser de Barenne, J.G. Die Strychninwirkung auf das Zentralnervensystem. III. Die segmenture Strychninvergiftung der dorsalen Rucken-marksmechanismen; ein Beitrag zur Dermatomerie der hinteren Extremität des Hundes. Folia neuro-biol., Lpz., 1911, 5: 342-359.
Dusser de Barenne, J.G., Die Strychninwirkung auf das Zentralnervensystem. I. Die Wirkung des Strychnins auf die Reflextaetigkeit der Intervertebralganglia. Folia neuro-biol., Lpz., 1910, 4:467-474.
Dusser de Barenne, J.G. Das Syndrom der Strychninvergiftung der dorsalen Ruckenmarkselemente: zugleich ein Beitrag sur Genese des Srtychnintetanus. Zbl. Physiol., 1911, 24: 840-842.
Dusser de Barenne, J.G. Die Strychninwirkung auf das Zentralnervensystem. II. Zur Wirkung des Strychnins bei lokuler Applikation auf das Rückenmark. Folia neuro-biol., Lpz., 1911, 5: 42-58.
Dusser de Barenne, J.G. and McCulloch, W.S. Physiological Delimitation of Neurones in the Central Nervous System. Amer. J. Physiol., 1939, 127: 620-628.
Dusser de Barenne, J.G. and McCulloch, W.S. Functional Organization in the Sensory Cortex of the Monkey (Macaca Mulatto). J. Neurophysiol., 1938, 1: 69-85.
Garol, H.W. Some Observations on Suppression of Electrical Activity of Areas 4 and 6. Amer. J. Physiol., 1940, 129: 361.
Dusser de Barenne, J.G. Sensori-motor Cortex and Thalamus Opticus, Amer. J. Physiol., 1937, 119: 263.
Dusser de Barenne, J.G. Sensori-motor Cortex and Optic Thalamus. XIth Cong. exp. Psychol., 1937, (1 p.).
Dusser de Barenne, J.G. and McCulloch, W.S. The Direct Functional Interrelation of Sensory Cortex and Optic Thalamus. J. Neurophysiol., 1938, 1: 176-186.
Dusser de Barenne, J.G. and McCulloch, W.S. Functional Interdependence of Sensory Cortex and Thalamus. Amer. J. Physiol., 1940, 129: 421.
Verhaart, W.J. C, and Kennard, Margaret A. Corticofugal Degeneration Following Thermocoagulation of Areas 4, 6 and 4-s in Macaca Mulatta. J. Anat., 1940, 74:239-254.
Dusser de Barenne, J.G. and McCulloch, W.S. Sensorimotor Cortex, Nucleus Caudatus and Thalamus Opticus. J. Neurophysiol., 1938, 1: 304-377.
Hines, Marion. The “Motor” Cortex. Bull. Johns Hopkins Hosp., 1937, 60: 313-336.
Dusser de Barenne, J.G. and McCulloch, W.S. Suppression of Motor Response upon Stimulation of Area 4-s of the Cerebral Cortex. Amer. J. Physiol., 1939, 126: 482.
Richter, C.P. and Hines, Marion. Increased Spontaneous Activity Produced in Monkeys by Brain Lesions. Brain, 1928, 61: 1-16.
For further research:
Wordcloud: activity animals area arm article-images barenne caudate caudatus connection core corpus cortex cortical dusser electrical electrodes emergence experiments face fig figure fired globus lesions localization mcculloch monkey motor movements name net nucleus observations pallidus point produced projections putamen question records response result sensory stimulation striatum strychninization suppression thalamus tremor windows
Keywords: Activity, Cells, Several, Point, Strychninization, System, Conduction, Electrodes, Author, Neuronography
Google Books: http://asclinks.live/2oer
Google Scholar: http://asclinks.live/xwvz
Jstor: http://asclinks.live/sbzv