CORTICAL ORIGIN AND DISTRIBUTION OF CORPUS CALLOSUM AND ANTERIOR COMMISSURE IN THE MONKEY (MACACA MULATTA)12 [45]
W.S. McCulloch and H.W. Garol
Introduction
The corpus callosum and anterior commissure constitute a fiber system larger than the sum of all systems ascending to and descending from the cerebral hemispheres. Anatomical studies(1) and retrograde(2) and Wallerian(3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14) degenerations following lesions of this commissural system have indicated only cortical origins and axonal distributions which were for a large measure cortical and roughly symmetrical.
Little is known of its function. Apart from symptoms referable to lesions of adjacent structures,(15) tumors, softenings and surgical sections(16) have failed to produce any characteristic disorders except, possibly, impairment of coordination of the hemispheres in complicated symbolic activity.(15) Even complete transection of the corpus callosum has not invariably prevented the spread of convulsions from one side of the body to the other.(16)
Physiological results without electrical records were equally negative.(17) Bishop was probably the first to demonstrate a function referable to the corpus callosum. At the symposium of the American Neurological Association in 1937 he indicated that “when both occipital poles are released from domination by deeper structures, by cutting above the geniculate bodies, the corpus callosum throws these cortical areas of the two hemispheres into exact synchronism.” In 1939 Erickson(18) found that section of the corpus callosum prevented the spread of electrical afterdischarge from one hemisphere to the other. In that same year, by stimulating one hemisphere electrically and recording from the other, Curtis and Bard(19) mapped the inter-hemispherical communications and, by sectioning the corpus callosum, they proved these to be by callosal fibers.
In 1937, while working on the functional organization of the sensory cortex of the monkey, Dr. Dusser de Barenne and one of the present authors had strychninized 1 sq. mm. of area 4 of one hemisphere and searched the other but found no strychnine spikes. When Curtis published his conclusive study the reason for the failure with strychnine was at once apparent, for the only strychninization had been in an area marked by him as having little or no callosal connection. One cannot hope, by local strychninization to discover any callosal connections which Curtis, by electrical stimulation, has not already disclosed, for strychnine acts only where synapses are present on nerve cells and causes disturbances propagated only in the normal direction,(20, 21) whereas we know no way to prevent electrical stimulation from producing antidromic disturbances and from exciting any axons passing through the stimulated area. It was, therefore, with the hope of clarifying and simplifying Curtis's findings, rather than of amplifying them, that the present study was undertaken.
Methods
All experiments were performed upon monkeys (Macaca mulatta) fully anaesthetized with Dial3 (0.45 cc. per kg., ½ intraperitoneal, ½ intramuscular). Both hemispheres were exposed widely, care being taken to preserve the cerebral circulation. For section of the corpus callosum, one side was prepared in advance by section of veins joining the brain to the superior longitudinal sinus. Thirty-six electrodes were placed on one hemisphere and connected, 6 at a time, to 5 channels of a Grass 6-channel inkwriter oscillograph. The arrangement was linear i.e., successive channels had one electrode in common. The sixth channel was used for roving electrodes on the other hemisphere, and moved to the site of successive strychninizations. These were of about 10 sq. mm. so that the major portion of one hemisphere could be strychninized in each animal. When no spikes appeared at any of the 36 electrodes the area symmetrical to the strychninization was examined thoroughly by moving one or more electrodes from place to place over the area in question. Additional electrodes on the hemisphere strychninized were used to identify the site.
Results
When one places on one hemisphere a small piece of filter paper moistened with a saturated solution of strychnine sulphate the surface of the cortex at the site of strychninization rapidly drifts negative and in less than half a minute there appear small fast negative fluctuations.(22) These cannot be detected except at the site of strychninization. Within about one minute a positive deflection precedes each negative fluctuation and a lesser and slower positive deflection follows. This is the fully developed strychstrychnine spike, and it is propagated as a recognizable strychnine spike to all other regions reached by axons from the area strychninized. It was by means of
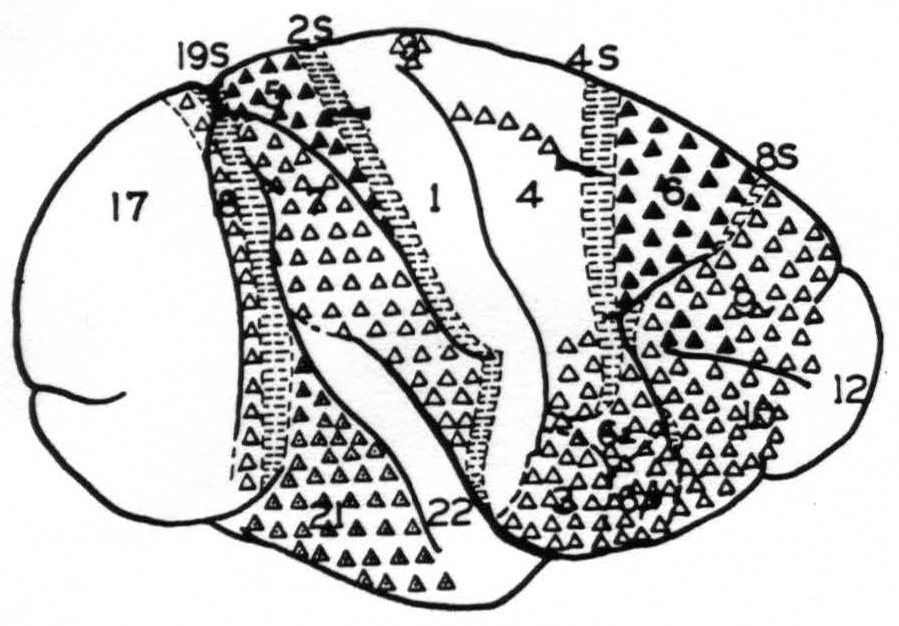
Figure 1. Map of the convexity of the hemisphere numbered in conformity with Brodmann's nomenclature except for those numbers followed by S to indicate that these areas are identified by their giving suppression of electrical activity. They are indicated by horizontal shading. △ = Projection to contralateral hemisphere at symmetrical focus only . ▲ = Projection to contralateral hemisphere at symmetrical and other foci. ◬ = Projection to contralateral hemisphere at symmetrical focus only which remains after section of the corpus callosum.
these strychnine spikes that the functional organization of the sensory and adjacent cortex(23) and of the occipital lobes were originally mapped and found to conform in the main to the cytoarchitectonic maps of Brodmann(24) and the Vogts(25, 26) The map so produced formed the basis for the present experiments in determining what physiologically unique area was strychninized on one hemisphere or recorded on the other. Figure 1 shows such a map of the convexity of the hemisphere on which are plotted schematically the results of the present investigation.
In these experiments many minute, rounded or slightly belated disturbances of the opposite hemisphere were encountered. These have been consistently excluded in making Fig. 1, for it was feared that these might be electrical spread from structures other than those of the cortex subjacent to the electrodes (e.g., fiber tract) or even post-synaptic disturbances. Thus, this diagram represents only the origin of indubitable commissural projections which pass from the cortex of convexity of one hemisphere to the cortex of the convexity of the other without relay.
None of the suppressor areas, 8s, 4s, 2s or 19s, gives rise to contralateral strychnine spikes, nor do areas 1, 12, 17 or 22. Area 9, except for a portion above the sulcus principalis, area 10, area 6a and b (face), area 4 (trunk, neck and face only), area 7, area 18 and area 21 give rise to contralateral disturbances restricted to foci symmetrical to the focus strychninized. Whereas part of area 9, above the sulcus principalis, area 6 (leg and arm) and area 5 (leg and arm) give rise to disturbances which are distributed to large areas of the opposite hemisphere. In fact, leg 6, arm 6, leg 5 and arm 5 give rise to disturbances of both pre-and postcentral sensory areas of the opposite hemisphere.
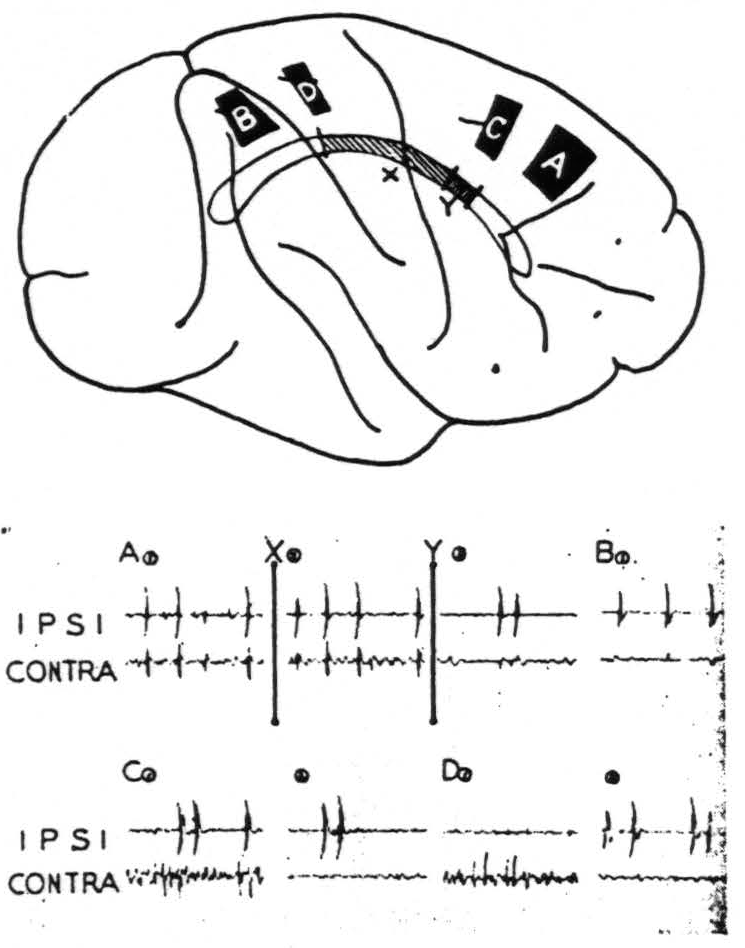
Figure 2. Feb. 28, 1941. Macaca mulatta. Dial. Diagram of the convexity of the right hemisphere indicating sites of strychninization. On this is projected the corpus callosum indicating two partial sections thereof, X and Y. All records were made with bipolar pickup electrodes at the site of strychninization (ipsi) and on the contralateral symmetrical point (contra). Record A① was made before section X; Record A②, after section X but before section Y; Record A③, after section Y. Records A③ and BA① show the minute residual disturbances of the contralateral hemisphere. Records C② and D② show suppression of the electrical activity of the contralateral hemisphere.
With one exception, section of the corpus callosum prevents the propagation of the strychnine spikes to the opposite hemisphere. Partial section prevents their propagation from part of the hemisphere. Figure 2 shows diagrammatically such an experiment. Following a large strychninization, A, of area 6 of one hemisphere which was projected to the opposite hemisphere (Record A1) the corpus callosum was partially sectioned (cf. X in the diagram and at time X in the record). The strychnine spikes came through to the opposite hemisphere unaltered (Record A2). The section was then extended (cf. Y in the diagram and at time Y in the record). Then there remained only very small spikes (Record A3). Thereafter several strychninizations occipital to strychninization A were performed on areas known to have callosal projections, but no spikes appeared on the opposite hemisphere
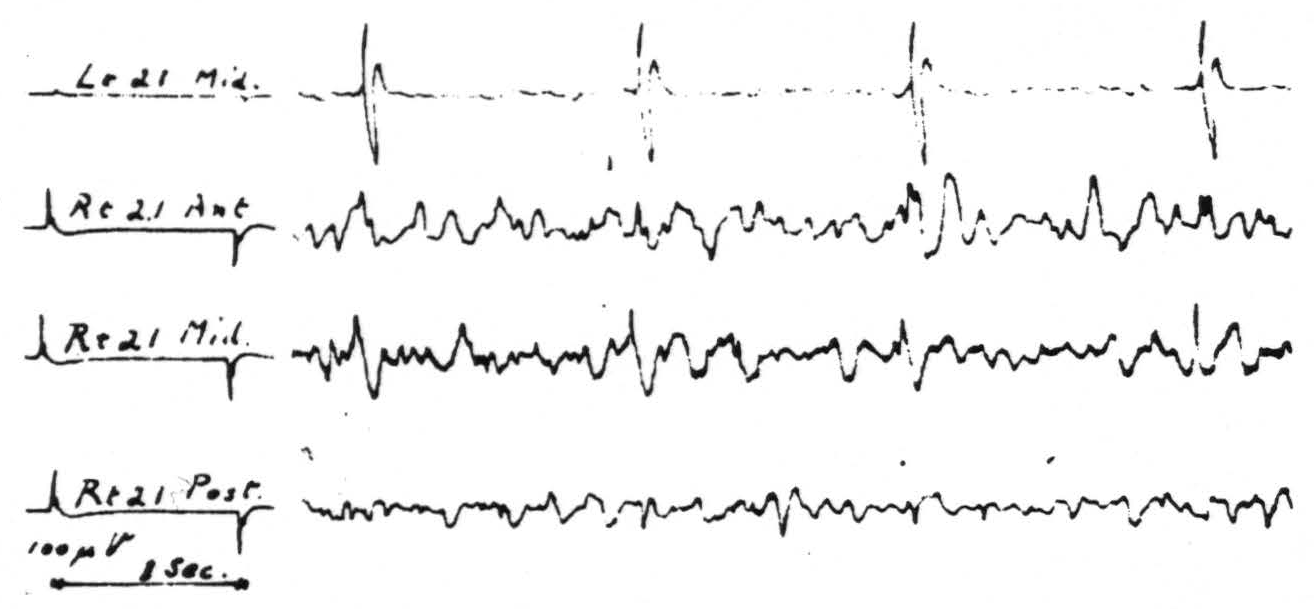
Figure 3. May 28, 1941. Macaca mulatta. Dial. Strychninization of entire left second temporal convolution, area 22, twenty minutes after complete transection of corpus callosum. Note strychnine spikes are largest in the record of the middle of the right second temporal convolution.
until strychnine was placed as far occipitally as B, which is in arm 7, when minute disturbances (Record B1) again appeared on the opposite hemisphere. Briefly, the section X+Y had interrupted almost all transmission via the corpus callosum from one sensory cortex to the other.
The exception mentioned above occurs in the case of area 21, the mid-temporal convolution. Strychninization in each part of this area resulted in typical spikes sharply restricted to the symmetrical focus of the opposite hemisphere. Section of the corpus callosum failed to prevent their occurrence. Figure 3 shows these spikes, as disclosed by the roving electrodes at the site and as recorded from the contralateral hemisphere. This strychninization covered practically the entire convolution and hence it is probably significant that the spikes on the contralateral hemisphere are larger and more constant in the middle third of the convolution than in either the anterior or posterior third. In this experiment the anterior commissure was intact.
In a second experiment it also was divided and thereafter no strychnine spikes were transmitted to the opposite hemisphere. Moreover, following section of the entire corpus callosum, electrical afterdischarge following stimulation of area 21 on one hemisphere spread to the same area of the other hemisphere, and this spread was also prevented by section of the anterior commissure.
Suppression of the electrical activity of one hemisphere by strychninization of 8s, 4s, 2s and 19s of the other hemisphere has also been observed. This suppression has been obtained after section of the corpus callosum. Records C1, C2, D1 and D2 of Fig. 2 instance such suppressions of the activity of the contralateral hemisphere after strychninizations of 4s and 2s which lie within an area all of whose callosal connections had already been severed.
Discussion
The known anatomy and physiology of the corpus callosum and anterior commissure and the known properties of the strychnine spike would lead one to expect at least all the positive findings of direct interhemispherical connection here presented. In fact, these constitute, so far as the callosal system is concerned, a partial confirmation of those of Curtis,(27) from which they differ only privatively. Due to the relatively large strychninizations and the large number of foci recorded following each strychninization it is not likely that the differences are due to oversight. It is possible that potentials which were regarded as of questionable significance, and so excluded from Fig. 1, would have been included by Curtis as small disturbances, but certainly the greatest reason for the differences must be sought in the dissimilarity of stimulation. Such positive findings of commissural connections as are obtained by its use indicate cell bodies which are situated in the area strychninized and give rise to axons extending to the site of appearance of the strychnine spikes. From the voltage of these spikes it is highly probable that many axons must participate, although it is impossible to say how many. Systematic exclusion of small and questionable disturbances may well have prevented indication of callosal connections which were comparatively scattered and not numerous. Thus Fig. 1 is in no sense a complete chart of interhemispherical connections but merely a diagram of the lateral aspect of one hemisphere on which are indicated those of its areas which give rise to numerous callosal axons ending in the cortex of the convexity of the opposite hemisphere. As less than one-sixth of the total cortex of one hemisphere is on the surface of the convexity Fig. 1 cannot represent more than one-sixth and may represent as little as one thirty-sixth of the total area giving axons to the commissural system.
The primary projection areas of vision and of somatic sensation are markedly deficient in callosal projections whereas so-called associational areas have them in abundance. Similarly when one looks at the primary motor area, 4, one finds these callosal connections restricted to areas controlling parts of the soma which are most frequently used symmetrically, and lacking from those representing the extremities which are moved independently. On the motor side of the sulcus centralis the greatest callosal projection arises from areas leg 6 and arm 6 whose stimulation yields more complex movements.
That section of the corpus callosum failed to prevent firing of one area 21 by the other, and that this firing occurred without time for relay in other
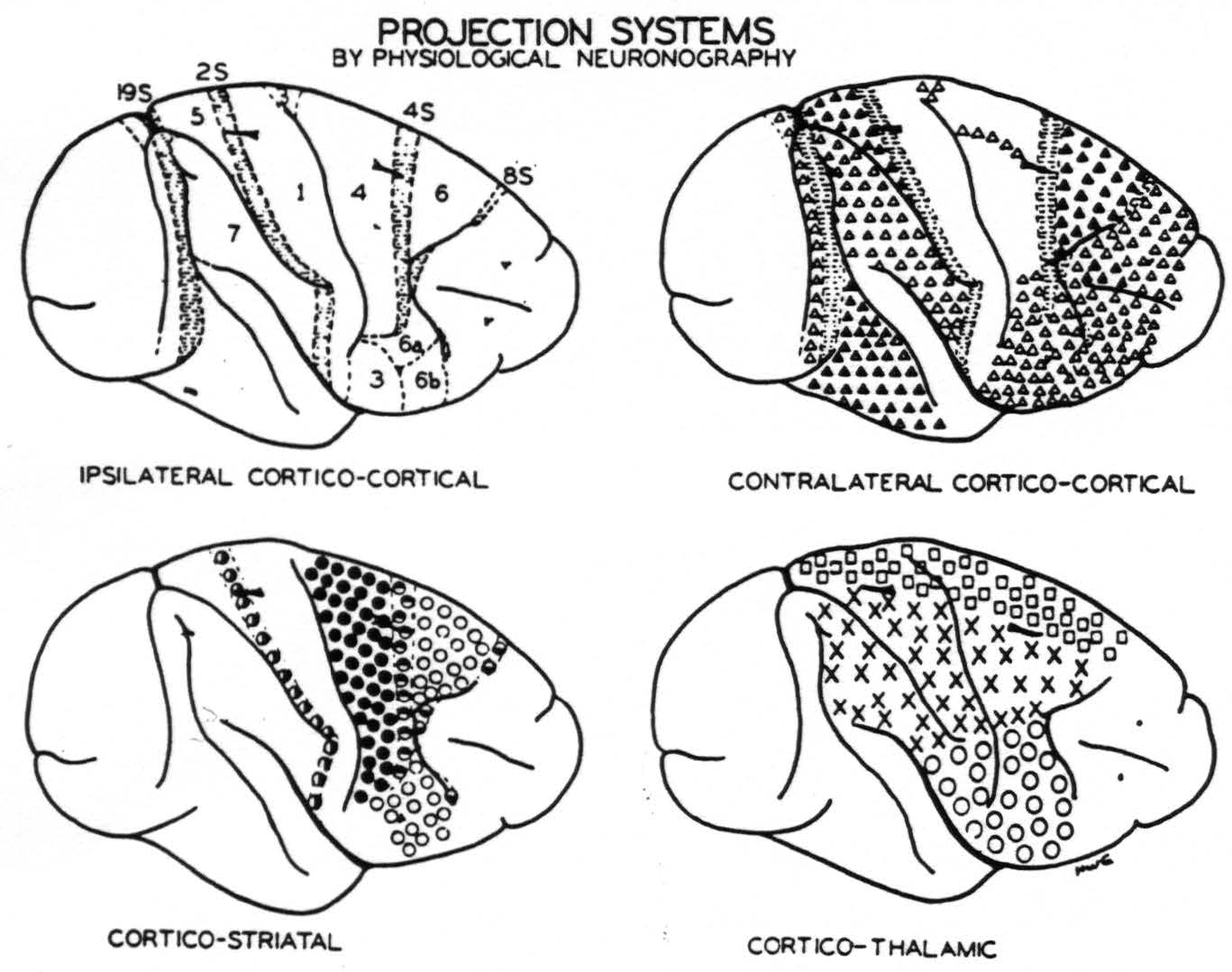
Figure 4. Numbers indicate portions of the sensory and adjacent cortex distinguished on the basis of their firing into other areas or being fired into from other areas of the same hemisphere except those numbers followed by S which yield suppression of electrical activity of the cortex. Horizontal shading indicates areas identified by suppression. △ = Projection to contralateral hemisphere at symmetrical focus only. ▲ = Projection ’ to contralateral hemisphere at symmetrical and other foci. ◬ = Projection to contralateral hemisphere at symmetrical focus only which remains after section of the corpus callosum. ◑ = Projection from area 2s to nucleus caudatus. ● = Projection to putamen. ◓ = Projection from area 4s to nucleus caudatus. ○ = Projection to putamen and to external segment of globus pallidus. ◒ = Projection from area 8s to nucleus caudatus. □ = Projection to leg nuclei of the thalamus. ✕ = Projection to arm nuclei of the thalamus. ◯ = Projection to face nuclei of the thalamus.
structures indicates that cells situated in area 21 have axons passing to the other hemisphere by a bridge other than the corpus callosum. Anatomical considerations by exclusion strongly indicate that this must be by way of the anterior commissure—a conclusion confirmed by failure to cross following section of the anterior commissure.
Since it is clear from previous work of this laboratory,(28) and from Erickson's study,(18) that electrical after discharge (and therefore the central disturbance in clonic seizures) spreads by neuronal paths it is to be expected that section of the corpus callosum alone would prevent the spread of many seizures from one side to the other. The remaining connection of the areas 21, by way of the anterior commissure, should suffice for the crossing if the disturbance spread to the temporal lobe on the first side. Erickson(18) had not investigated these regions from, and to which crossing occurs via the anterior commissure. As shown by the present findings of spread of after discharge, these may play a significant role in those patients of whom Van Wagenen(16) reports that they had seizures recurring bilaterally after section of the corpus callosum. Thus, apart from its surgical implication, this finding concerning the anterior commissure is significant in explaining the apparent contradiction between Erickson's conclusions and Van Wagenen's reports.
Two new findings with respect to the suppressor areas—(i) that suppression of electrical activity is bilateral, (ii) that it remains bilateral after section of the corpus callosum—are in harmony with conclusions from previous experiments.(29, 30) Since the path responsible for suppression of electrical activity is known to be subcortical and the effect is bilateral, and since suppression of motor response(31, 32) to cortical stimulation is necessarily subcortical, and bilateral, the third finding is not surprising—namely, that the areas (8s, 4s, 2s and 19s) responsible for these suppressions give rise to no demonstrable callosal projection.
Curtis has already called attention to the failure of the callosal system to correspond to cytoarchitectonic or somatotopic subdivision of the cortex.(27) Figure 4 amplifies this point by contrasting four maps of the convexity of the hemisphere as so far revealed by physiological neuronography. The upper left shows how that cortex can be divided on the basis of corticocortical connections.(25, 23, 28, 33, 34) The suppressor areas (8s, 4s, 2s and 19s) lack such connections. The upper right shows the principal origins of the corpus callosum and anterior commissure. Again the suppressor areas lack those connections. The lower left shows the areas separated on the basis of their projection to basal ganglia.(29, 30) Here all half-filled circles indicate connections to the nucleus caudatus. All come from the suppressor areas, and stimulation of the nucleus caudatus yields suppression. The lower right indicates origins of the essentially somatotopic projections to the leg, arm and face nuclei of the thalamus.(35, 36) Thus, this figure reveals the unique origin of each of these systems. The corpus callosum and anterior commissure are no exceptions.
Conclusions
Based on local strychninization of one hemisphere and electrical records of the other, i.e., by physiological neuronography, a new map of the convexity of the cerebral hemisphere of Macaca mulatta has been prepared to show the origins of the corpus callosum and anterior commissure and to indicate (i) those origins whose interhemispherical projections are dispersed to many areas of the convexity of the other hemisphere, and (ii) those whose projections are restricted to symmetrical foci.
The former spring from a part of area 9 above the sulcus principalis and from areas 5 and 6 of the leg- and arm-subdivisions. The latter have been found to arise from many parts of the frontal pole, from face 6, from the trunk and face portions of area 4 and from areas 18 and 21.
The last of these restrictedly symmetrical projections, i.e., that between the areas 21, is unique, for it alone remains after section of the corpus callosum, provided the anterior commissure is intact.
Suppression of electrical activity of the cortex by strychninization of 8s 4s, 2s or 19s has been found to be always bilateral, even after section of the corpus callosum to which these areas contribute few or no axons.
Footnotes
References
Beevor, C. E. On the course of the fibres of the cingulum and the posterior parts of the corpus callosum and fornix in the marmoset monkey. Philos.Trans., 1892, 182B: 135-200.
Pines, L. J., and Maiman, R. M. Cells of origin of fibers of corpus callosum. Experimental and pathological observations. Arch. Neurol. Psychiat., Chicago, 1939, *42: *1076-1082.
Biemond, A. Ueber den Verlauf der okzipitalen Balkenfasern und eine neue Verbindung des Cingulums beim Java-Affen. Proc. Acad. Sci. Amst., 1932, 35: 1166-1170.
Mettler, F. A. Connections of the auditory cortex of the cat. J. comp. Neurol., 1932, 55:139-183.
Mettler, F. A. Corticofugal fiber connections of the cortex of Macaca mulatta. The occipital region. J. comp. Neurol., 1935, 61: 221-256.
Mettler, F. A. Corticofugal fiber connections of the cortex of Macaca mulatta. The frontal region. J. comp. Neurol., 1935, 61:509-542.
Mettler, F. A. Corticofugal fiber connections of the cortex of Macaca mulatta. The parietal region. J. comp. Neurol., 1935, 62: 263-291.
Mettler, F. A. Corticofugal fiber connections of the cortex of Macaca mulatta. The temporal region. J. comp. Neurol., 1935-36, 63: 25-47.
Mingazzini, G. Der Balken. Eine anatomische, physiopathologische und klinische Studie. Berlin, J. Springer, 1922, xv, 212 pp.
Muratoff, W. Secundäre Degeneration nach Durchschneidung der Balkens. Neurol Zbl., 1893, 12: 714-729.
Muratoff, W . Secundäre Degeneration nach Zerstörung der motorischensphäre des Gehirns. Arch. Anat. Physiol., Lpz., 1893, p. 99-116.
Poljak, S. An experimental study of the association callosal, and projection fibers of the cerebral cortex of the cat. J. comp. Neurol., 1927, 44: 197-258.
Sunderland, S. The distribution of commissural fibers in the corpus callosum in the macaque monkey. J. Neurol. Psychiat., London, 1940, 3: 9-18.
Valkenburg, C. T. van. Experimental and pathologic-anatomical researches on the corpus callosum. Brain, 1913, 36: 119-165.
Sweet, W. H. Seeping intracranial aneurysm simulating neoplasm. Syndrome of the corpus callosum. Arch. Neurol. Psychiat., Chicago, 1941, 45: 86-104.
Van Wagenen, W. P., and Herren, R. Y. Surgical division of commissural pathways in the corpus callosum. Arch. Neurol. Psychiat., Chicago, 1940, 44: 740-759.
Kennard, Marcaret A., and Watts, J. W. The effect of section of the corpus callosum on the motor performance of monkeys. *J. nerv. ment. Dis., *1934, 79: 159-169.
Erickson, T. C. The spread of epileptic discharge. An experimental study of the after-discharge induced by electrical stimulation of the cerebral cortex. Arch. Neurol. Psychiat., Chicago, 1940, 43 : 429-452.
Curtis, H. J., and Bard, P. Intercortical connections of the corpus callosum as indicated by evoked potentials. Amer. J. Physiol., 1939, 126: P473.
Dusser de Barenne, J. G. Die Strychninwirkung auf das Zentralnervensystem. IV. Theoretische Betrachtungen. Folia neuro-biol., 1912, 6: 277-286.
Dusser de Barenne, J. G., and McCulloch, W. S. Physiological delimitation of neurones in the central nervous system. Amer. J. Physiol., 1939, 127:620-628.
Dusser de Barenne, J. G., and McCulloch, W. S. Kritisches und Experimentelles zur Deutung der Potentialschwankungen des Elektrocorticogramms. Z. ges. Neurol. Psychiat., 1938,162:815- 823.
Dusser de Barenne, J. G., Garol, H. W., and McCulloch, W. S. Functional organization of sensory and adjacent cortex in the monkey. J. Neurophysiol., 1941, 4: 324-330.
Brodmann, K. Vergleichende Lokalisationslehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues. Leipzig, J. A. Barth, 1909, xii, 324 pp.
Bonin, G. Von, Garol, H. W., and McCulloch, W. S. Functional organization of occipital lobe. Anat. Rec., 1941, 79 (Suppl.) : 10.
Curtis, H. J. Cortical potentials mediated by the corpus callosum. Amer. J. Physiol., 1940,129:* 341-342.
Curtis, H. J. Intercortical connections of corpus callosum as indicated by evoked potentials. J. Neurophysiol., 1940, 3: 407-413.
Dusser de Barenne, J. G., and McCulloch, W. S. Functional organization in the sensory cortex of the monkey (Macaca mulatto). J. Neurophysiol., 1938, 1: 69-85.
Dusser de Barenne, J. G., Garol, H. W., and McCulloch, W. S. Physiological neuronography of the cortico-striatal connections. Res. Publ. Ass. nerv. ment. Dis., In press.
Dusser de Barenne, J. G., and McCulloch, W. S. Sensorimotor cortex, nucleus caudatus and thalamus opticus. J. Neurophysiol., 1938, 1: 364-377.
Dusser de Barenne, J. G., and McCulloch, W. S. Suppression of motor response upon stimulation of area 4-s of the cerebral cortex. Amer. J. Physiol., 1939, 126: P482.
Dusser, de Barenne, J. G., and McCulloch, W. S. Suppression of motor response from area 4 by stimulation of area 4s. J. Neurophysiol., 1941, 4: 311-323.
Dusser de Barenne, J. G., McCulloch, W. S., and Ogawa, T. Functional organization in the face-subdivision of the sensory cortex of the monkey (Macaca mulatto). J. Neurophysiol., 1938, 1: 436-441.
Garol, H. W. Some observations on the suppression of electrical activity of areas 4 and 6. Amer. J. Physiol., 1940,129: 361.
Dusser de Barenne, J. G. Sensori-motor cortex and thalamus opticus. Amer. J. Physiol., 1937,119: 263.
Dusser de Barenne, J. G. Sensori-motor cortex and optic thalamus. Xlth Congr. exp. Physiol., 1937, (1 p.).
Curtis, H. J. An analysis of cortical potentials mediated by the corpus callosum. J. Neurophysiol., 1940, 3: 414-422.
Vogt, C., and Vogt, O. Allgemeinere Ergebnisse unserer Himforschung. J. Psychol. Neurol., Lpz., 1919, 25: 277-462.
For further research:
Wordcloud: Activity, Anterior, Area, Arm, Axons, Barenne, Callosal, Callosum, Commissure, Connections, Contralateral, Convexity, Corpus, Cortex, Curtis, Disturbances, Dusser, Electrical, Electrodes, Except, Figure, Findings, Following, Functional, Give, Hemisphere, Indicate, Leg, Macaca, Map, McCulloch, Monkey, Mulatta, Neurol, Opposite, Origins, Physiol, Prevent, Projection, Record, Section, Site, Spikes, Spread, Stimulation, Strychninization, Suppression, Symmetrical, System
Keywords: Callosum, Hemispheres, Commissure, Mulatta, System, Looms, Sections, Origin, Commisure, Structures
Google Books: http://asclinks.live/2xo1
Google Scholar: http://asclinks.live/80jz
Jstor: http://asclinks.live/gawc