SUPPRESSION OF MOTOR RESPONSE OBTAINED FROM AREA 4 BY STIMULATION OF AREA 4s12 [43]
J.G. Dusser de Barenne and W.S. McCulloch
Introduction
In 1934 during the investigation of facilitation and extinction of motor response to cortical stimulation (1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11) due to antecedent stimulation of neighboring foci a new phenomenon appeared when the antecedent stimulation was in either the frontal part of area 4 or in the posterior part of area 6. When stimulation of this region preceded by several minutes the testing stimulation of area 4 no response to that stimulation of area 4 was obtained. Careful examination of the records showed frequently a slight lowering of the base line, suggesting a relaxation of the extremity. During mapping of the functional organization of the sensory cortex,(12) the region from which the new phenomenon could be elicited was discovered to give, upon local strychninization within it, a diminution of the electrical activity of area 4. The area yielding these phenomena, “suppression of motor response” and “suppression of electrical activity,” was designated area 4s and was found to coincide with the strip of cortex from which Dr. Marion Hines obtained, by electrical stimulation, a cessation of movements and a relaxation of contracted muscles” and ablation of which by the same author produced spasticity(13)
In a previous publication on the functional organization of the sensory cortex(12) it was shown that the “firing” of one area by another was dependent upon cortico-cortical connections which remained intact when the cortex was severed from deeper structures, by deep undercutting, whereas, in a subsequent publication,(14) it was demonstrated that the suppression of electrical activity of area 4 by strychninization of area 4s was dependent upon a one-way circuit from area 4s to the nucleus caudatus, thence to the thalamus and so to the cortex. As it was hoped, at that time, that the analogous phenomenon, the suppression of motor response, might readily be traced either in the cortico-cortical system or, more probably, through the corresponding deeper structures the publication of a full description of the phenomenon was delayed until the experiments could be performed to demonstrate its circuit. This proved more difficult than was expected, wherefore it was deemed wisest to write the present article in which the phenomenon is fully described, the distinction between it and the extinction of motor response made clear and, finally, the attempts to trace its path through the central nervous system are recorded together with the reason for each experiment.
Methods
All experiments were performed upon monkeys (Macaca mulatta) anaesthetized with Dial3 (0.45 cc. per kg. body weight, ½the dose intraperitoneal, ½intramuscular). The cortex was exposed and stimulated with thyratron impulses delivered through Ag-AgCl electrodes. Responses were recorded isotonically on a smoked paper kymograph. When necessary these procedures were supplemented by recording the electrical activity of various structures of the central nervous system. This was done with moving paper camera and 4-channel Westinghouse oscillograph with appropriate amplifiers.
Lesions were made chiefly by a small blade mounted on a mechanically controlled needle which could be thrust into the brain at any required angle to any required depth, there rotated and then returned to the original position before retraction, thus minimizing the lesion of approach.
Brains with lesions were taken up into a sarcophagus with agar (of approximately the same consistency as the brain), extruded against an angular mount, sectioned with a spatula into slices either 1 or 2 mm.,(15) and photographed.
Results
Stimulation of the cerebral cortex with a manually applied electrode by well controlled thyratron impulses discloses that the threshold rises fairly
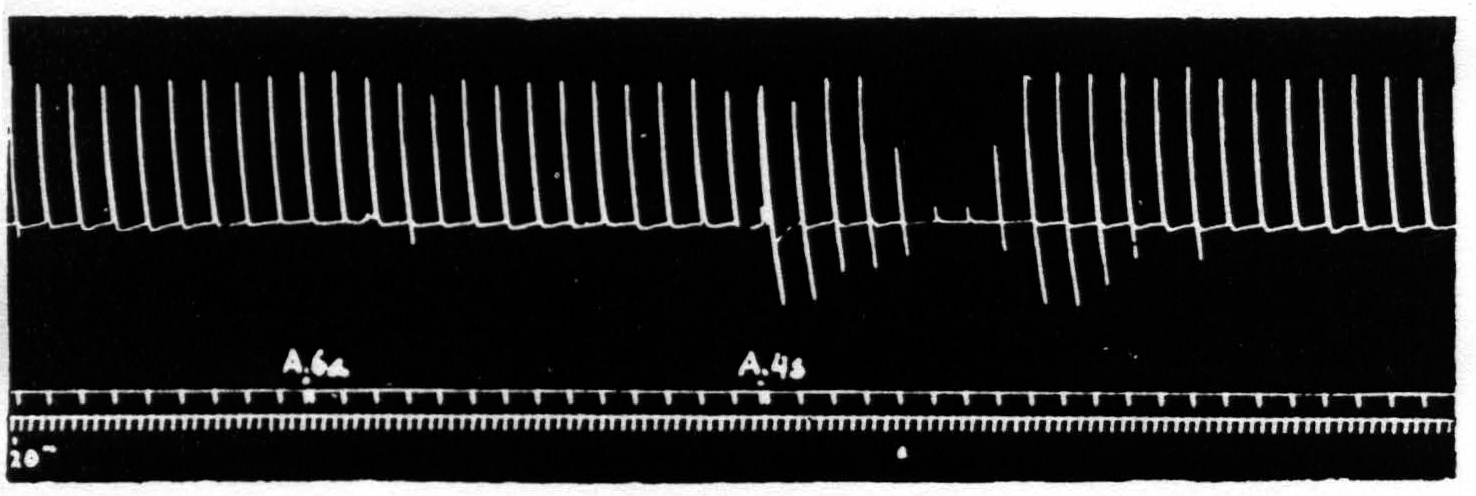
Figure 1. Sept. 27, 1938. Macaca mulatta. Dial. Electrical monopolar stimulation (Thyratron) of A4 focus once every minute (5 sec.-0.6 µF.-40 per sec.-V.D. 1200). Extension of wrist. Electrical stimulation of A6a focus gives no suppression. Electrical stimulation of A4s focus (6 sec.-1 µF.-40 per sec.-V.D. 7000) gives suppression. Time line = 20 sec.
abruptly as one passes from the posterior to the more anterior part of area 4. This change is encountered in about the middle of area 4 and is of the order of a 100 per cent rise in threshold. If the impulses are relatively long this threshold is not very different from that of area 6, but between area 4 and area 6 lies a narrow band of cortex (2 or 3 mm. wide and running dorsoventrally through the anterior part of the superior precentral sulcus) where stimulation fails to elicit any contractions of muscles until such voltages are reached that spread of current must have involved the adjacent areas. This band of cortex from which no contraction of muscles is elicited is the strip of Marion Hines,(13, 16) area 4s, i.e., the strip from which one obtains a suppression of electrical activity of area 4. But the stimulation of area 4s, although it fails to elicit contraction, has (as Marion Hines has shown) definite effects which indicate that it has excited the cortex.
Figure 1 shows the effect with which this paper deals, namely, the suppression of motor response to electrical stimulation of a motor focus of area 4 by antecedent stimulation of area 4s. If one stimulates a focus of area 4 once a minute with such a stimulus that neither facilitation nor extinction disturbs the amplitude of the successive responses and then one stimulates area 4s the responses to the area 4 stimulation diminish or disappear.
Figure 2 shows that this suppression of motor response can be brought about by chemical or mechanical as well as by electrical stimulation of area 4s, and therefore cannot be due to inadvertent stimulation by spread of current to area 4 or to subjacent fiber tracts—a conclusion confirmed by thermocoagulation of the entire thickness of the cortex which prevents suppression.
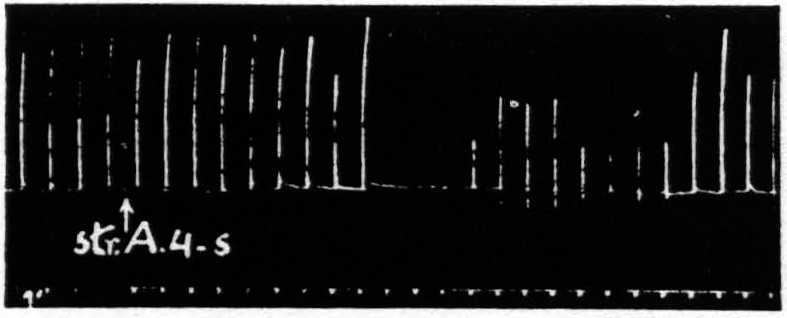
Figure 2. April 13, 1939. Macaca mulatta. Dial. Electrical stimulation once every minute of focus of A4 for extension of fingers (5 sec.-0.5 µF.-40 per sec.-V.D. 3600). Strychninization of A4s. Note 9 min. latency, and bimodal suppression.
Several characteristics of this suppression of motor response are noteworthy: (i) The suppression has a remarkable latency after electrical stimulation. The duration of latency most frequently encountered is 4 min., but latencies as short as 2 min. or as long as 12 min. have been obtainable by use of light anesthesia and strong stimulation of 4s for the shorter, and deep anesthesia and weak stimulation of 4s for the longer latencies. After mechanical stimulation the latency is usually short, whereas after strychninization, measured from the moment of application, the latencies are usually from 12 to 20 min. (ii) The suppression lasts several minutes. Suppression of motor response to 6 or 7 stimulations at one-minute intervals is ordinarily encountered. With weak stimulation of area 4s the duration is usually shorter. Suppressions lasting 20 to 30 min. are not uncommon with strong stimulation of area 4s in deeply narcotized animals. (iii) The suppression, if long, is frequently bimodal. Longer suppressions are occasionally trimodal. (Cf. Fig. 2, 5 and 7.) (iv) The suppression is accompanied by a change in muscle tension. During the suppression, sometimes starting before noticeable change in amplitude and sometimes lasting after amplitude has returned to its original level, there is a slight fall in the base line provided there is tension in the direction of gravity in the thread to the tambour in the resting position, thus indicating a loss of tension of the muscles supporting the extremity. Moreover, the last responses before, and several after the diminution (or absence) of amplitude look atonic and frequently graph the relaxed condition of the muscles by falling past the resting position. (Cf. Fig. 1 and 2.) (v) The suppression cannot be reinduced immediately. As these experiments were tedious attempts were made to find out how soon it was advisable to attempt to repeat the experiment. After a prompt, short suppression induced by mechanical stimulation of 4s, 15 to 20 min. suffices but with a typical 4-minute latency, 6-minute suppression following electrical stimulation of 4s, the experiment usually fails if attempted within 35 min. and succeeds at the end of 45 min.
The second group of findings are those that separate the suppression of motor response from the extinction of motor response to which it bears only a superficial resemblance.
The first, and obvious, difference is that the only part of the so-called motor cortex from which the suppression of motor response to cortical stimulation of any motor focus of area 4 can be obtained is area 4s; whereas extinction is obtained maximally by antecedent stimulation of one and the same focus and can be obtained by antecedent stimulation elsewhere only if the disturbance (notably, in the form of an after-discharge) actually involves the structures excited by the test stimulation. Whether such is the case when 4s stimulation produces unresponsiveness to stimulation of area 4 can be examined by electrical recording of the activity of the focus of area 4 to which the test stimulus is to be applied. The experiment proves that suppression of motor response can be elicited with no trace of after-discharge in any part of the cortex.
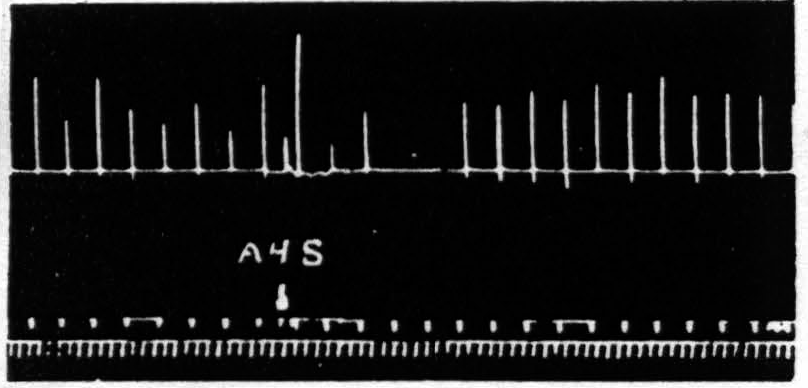
Figure 3. Oct. 4, 1938. Macaca mulatta. Dial. Flexion of fingers. Stimulation of A4s (5 sec.-1 µF.-50 per sec.-V.D. 6600) at posterior margin of A4s. Note small primary response. The responses to the following A4 stimulation (5 sec.-0.5 µF.-50 per sec.-V.D. 3600) show (i), facilitation, (ii), extinction, (iii), return, then suppression.
The second difference between these phenomena is also essentially spatial. Except where antecedent stimulation has been so extreme as to initiate an after-discharge so violent as to spread across the functional boundaries between the face-, arm- and leg-subdivisions, extinction has always remained localized to the subdivision stimulated; whereas the suppression of motor response to stimulation of a motor focus of, let us say, A4 can be elicited by antecedent stimulation of L4s, A4s, F4s on the same hemisphere and even, although less markedly, by stimulation of 4s on the opposite hemisphere.
The third difference is temporal. Except for the belated components of extinction due to the slow progress of a persistent after-discharge, which have been carefully excluded, extinction lasts a matter of one or two minutes at the most, whereas suppression has a latency of some four minutes. To make perfectly certain of the temporal separation in a single suppression one has only to place the 4s electrodes on the posterior margin of area 4s of the same subdivision as that of the area 4 focus used in testing and to increase the voltage of the area 4s stimulus sufficiently so that spread of current stimulates the more anterior part of area 4. One obtains then, by proper timing, facilitation to the first test stimulus, extinction to the second, no change to the third and suppression to the subsequent stimulations for the next several minutes. Figure 3 demonstrates this finding.
It is appropriate here to record another finding. At the suggestion of John Hamilton of this laboratory that the suppression of motor response from what was intended for stimulation of area 4s might be merely the effect of simultaneous stimulation of area 4 and area 6, two monopolar electrodes were placed one in area 6, immediately anterior to 4s, and the other in area 4, immediately posterior to area 4s and these were connected to the thyratron used for 4s stimulation. These monopolar electrodes were scarcely 3.5 mm. apart. Yet, even with slightly higher values of stimulation than sufficed in area 4s no suppression of motor response occurred. Instead, there was a primary response to the conjoined stimulation, followed by the facilitation and subsequent extinction of response to area 4 stimulation.
Inasmuch as the suppression of motor response was not restricted either to responses elicited from one part of the cortex, or to responses of a single
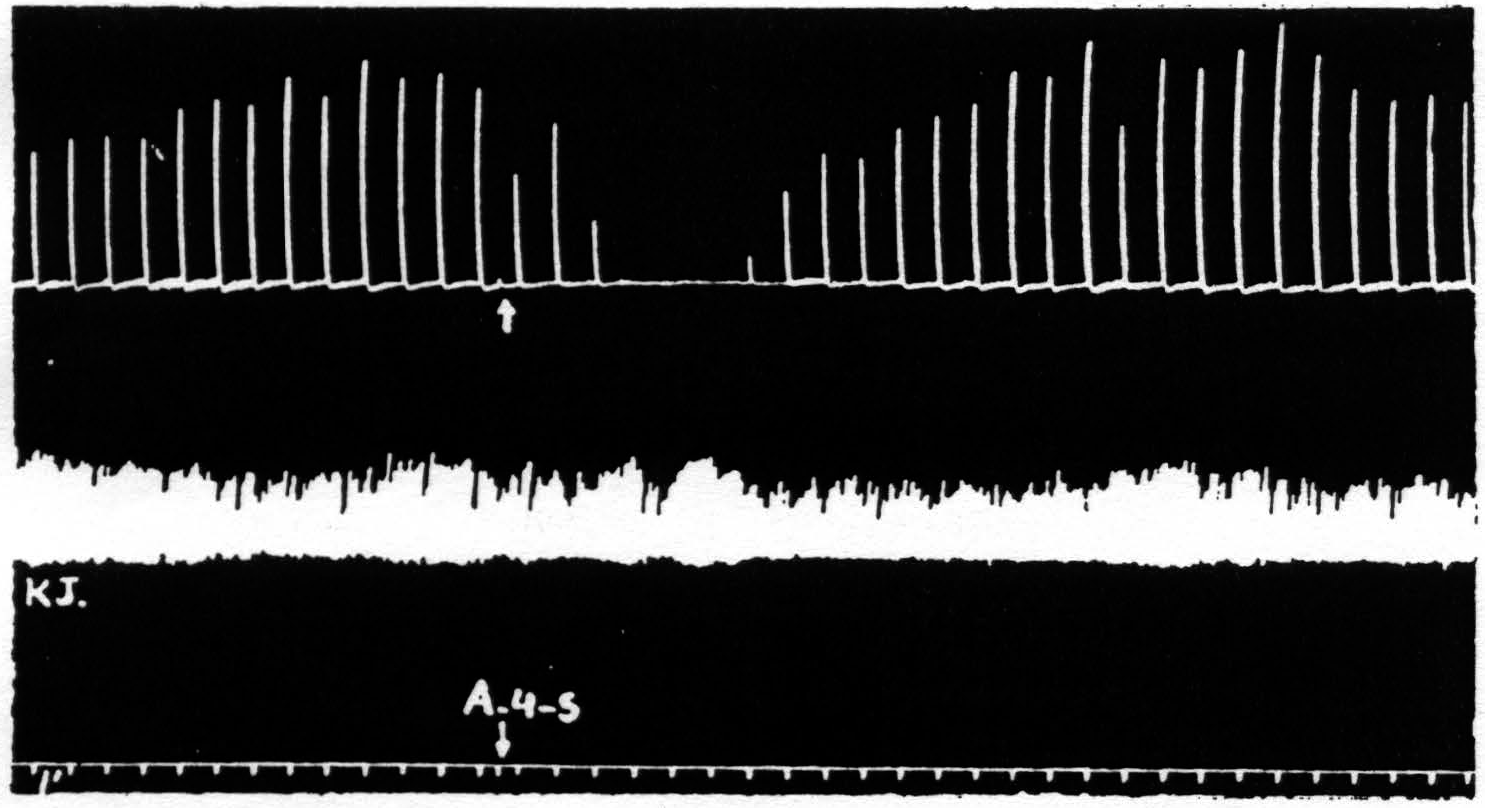
Figure 4. March 7, 1939. Macaca mulatta. Dial. A4 =5 sec.-0.5 µF.-50 per sec.-V.D. 800. A4s =5 sec.-1 µF.-50 per sec. V.D.1600. Time line =1 min. Upper record, extension of wrist in response to stimulation of A4 focus. Lower record, K.J. unaffected during suppression of motor response to cortical stimulation.
part of the body, the question at once arose as to whether it might not be due to an induced systemic alteration of circulation, respiration or metabolism which, in turn, produced a completely generalized rise in threshold either throughout the central nervous system or throughout the musculature. To settle this question it seemed simplest to examine a response which was initiated by other than cortical stimulation. The knee jerk in response to weak mechanical stimulation of the patella tendon every 4 sec. was, therefore, recorded before, during and after a suppression of motor response. Figure 4 shows the result of this experiment. The knee jerk is not altered.
Thus one has to look for a disturbance in restricted parts of the central nervous system and to attempt to trace its course from the area stimulated —area 4s—to the structures involved in the response to the testing stimulation of area 4. The simplest possible path which might be involved is a cortico-cortical connection from area 4s to area 4. However, section by a deep incision down to the nucleus caudatus does not prevent suppression. Figure 5 shows the suppression elicited after such a section.
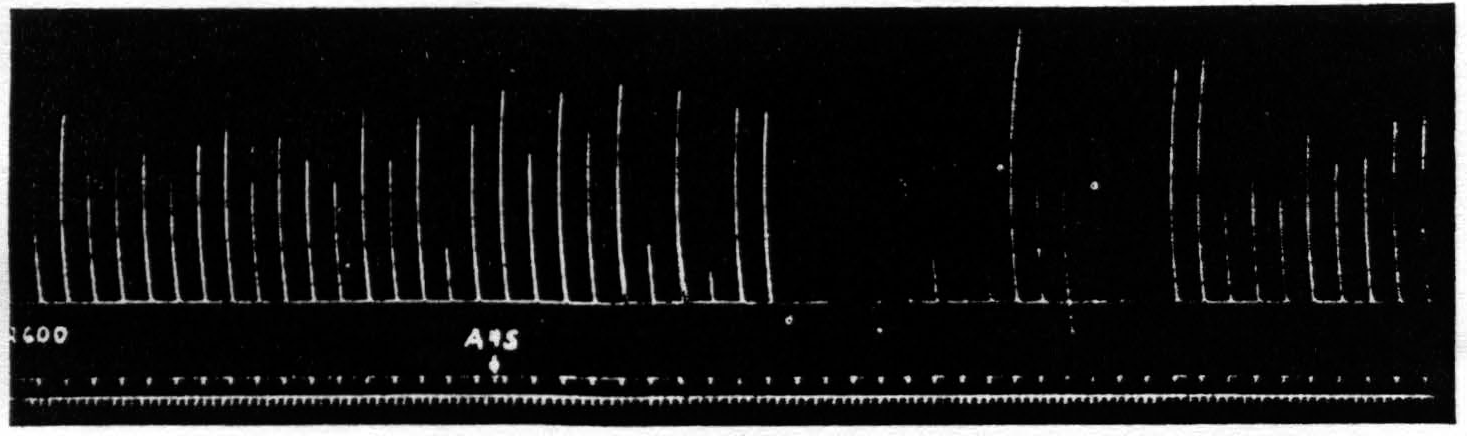
Figure 5. Oct. 10, 1938. Macaca mulatta. Dial. Suppression of motor response at 9:05 P.M. (A4s =5 sec.-1 µF.-40 per sec.-V.D. 5600. A4 =5 sec.-0.5 µF.-40 per sec.-V.D. 2600) subsequent to large lesion of nucleus caudatus at 5:00 P.M. and incision 13 mm. deep between A4s and A4 at 7:40 P.M.
Since one had to look, therefore, into cortico-subcortical connections, and since among these the cortico-caudate projection was known to be necessary for the suppression of electrical activity of area 4 upon strychninization of area 4s, an attempt was made to pith the nucleus caudatus by a frontal approach. This lesion prevented the suppression, but at autopsy, although the nucleus caudatus was extensively injured, the blade had been at such an angle as to sever the descending fibers from area 4s, and no conclusion could be drawn as to which part of the lesion was significant in the prevention of suppression.
Therefore, a small undercutting of 4s was made. This simple lesion prevented suppression and demonstrated that the disturbance necessary for suppression required a descending track. This is the direct proof of what one could never legitimately infer by exclusion from the results of severance of cortico-cortical connections.
Next a lesion was made in the nucleus caudatus without injury to the cortico-caudate fibers external to the caudate, by approaching it at another angle. The suppression of motor response was obtained again. Figure 6 shows the lesion and the suppression. This lesion is rather small but was sufficient to prevent any suppression of electrical activity of area 4 upon strychninization of area 4s even after the suppression of motor response had again been elicited after the lesion. Larger lesions of the nucleus caudatus also failed to prevent the suppression of motor response. One is therefore forced to conclude that the nucleus caudatus which is necessary for the suppression of electrical activity is either not necessary for the analogous suppression of motor response or that remaining parts of the nucleus caudatus, although insufficient for suppression of electrical activity of area 4, are still sufficient for suppression of motor response.
It seemed advisable in any case to exclude other parts of the same system,
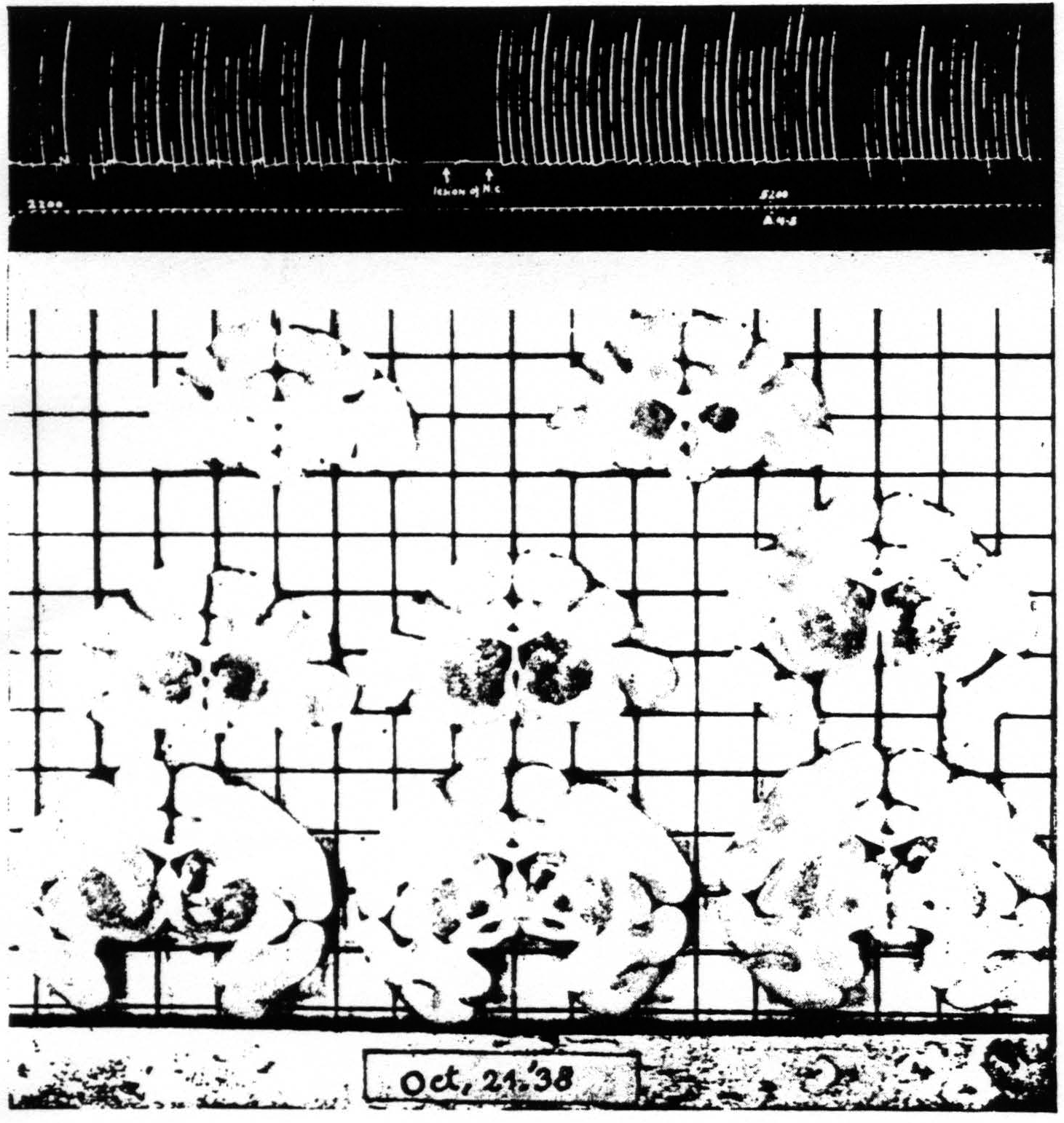
Figure 6. Oct. 21, 1938. Macaca mulatta. Dial. Extension of fingers. Continuous record. “Release” of motor response and suppression of motor response after lesion of nucleus caudatus which prevented suppression of ECG of A4. A4 =5 sec.-0.6 µF.-40 per sec.-V.D. 2200. A4s =6 sec.-1 µF.-40 per sec.-V.D. 5200.
i.e., basal ganglia, by a similar procedure. Therefore a massive lesion was made in the putamen and the suppression was again elicited. The same obtains with respect to the globus pallidus and the thalamic nuclei (see Fig. 7).
As the descending system from the cortex anterior to area 4 is said to be traceable by myelin degeneration as far as the pons varolii but not below it and as it is said to be connected there with the substantia nigra and the pontine nuclei,(17, 18) these possible paths were investigated.
First, to exclude any influence of the opposing half of the brain, the corpus callosum, anterior commissure and commissura mollis were divided. Suppression remained. Second, the substantia nigra was pithed bilaterally.
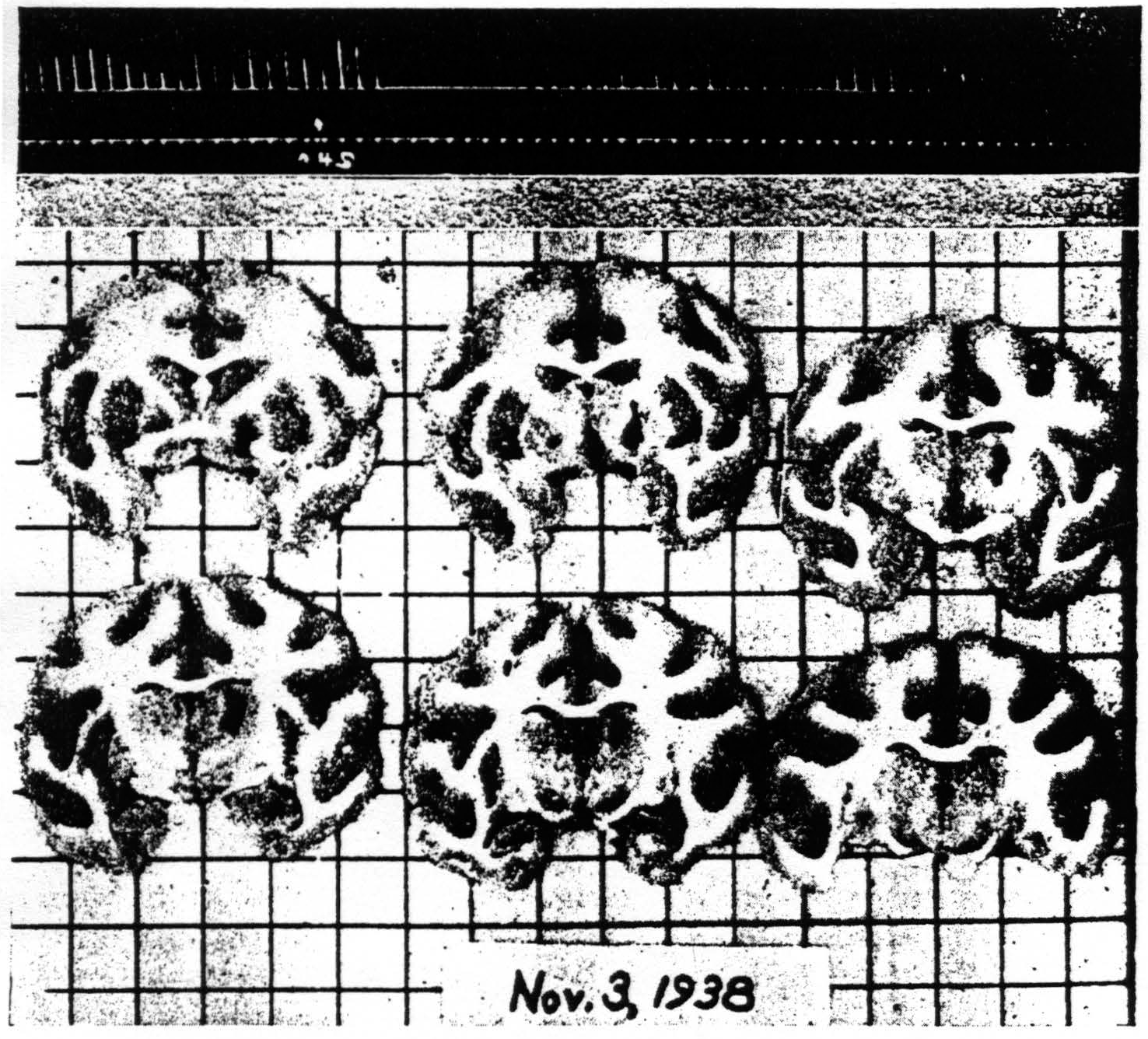
Figure 7. Nov. 3, 1938. Macaca mulatta. Dial. Flexion of left wrist. Suppression of motor response present after lesion (at 8:40 P.M.) of palladum and thalamic nuclei (arm and leg) A4 =5 sec.-0.5 µF.-40 per sec.-V.D. 2500. A4s =5 sec.-1 µF.-40 per sec.-V.D. 7500. (10:59 P.M.). Time line —1 min.
Figure 8 shows the results of the operation. Suppression of motor response could still be elicited from area 4s. Finally, the cerebellum was removed. Figure 9 shows the subsequent suppression of motor response.
These statements must not be taken to mean that suppression could always be obtained in each type of experiment. In fact, an experiment sometimes had to be performed on three or even four animals before it could be established that suppression could still he elicited after a lesion of the type
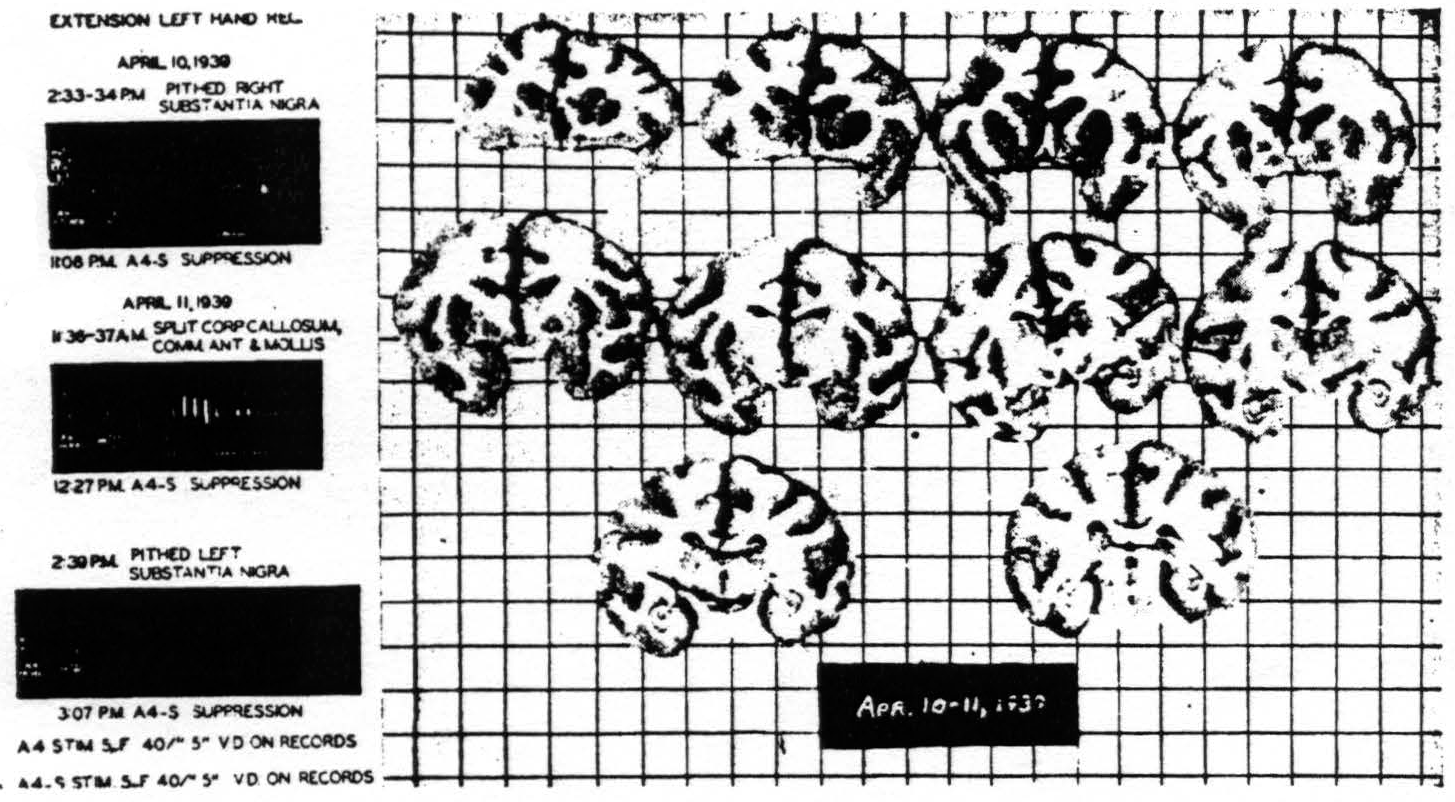
Figure 8. Macaca mulatta. Dial. Suppression of motor response after bilateral lesions of substantia nigra and splitting of brain. For parameters of stimulation see records.
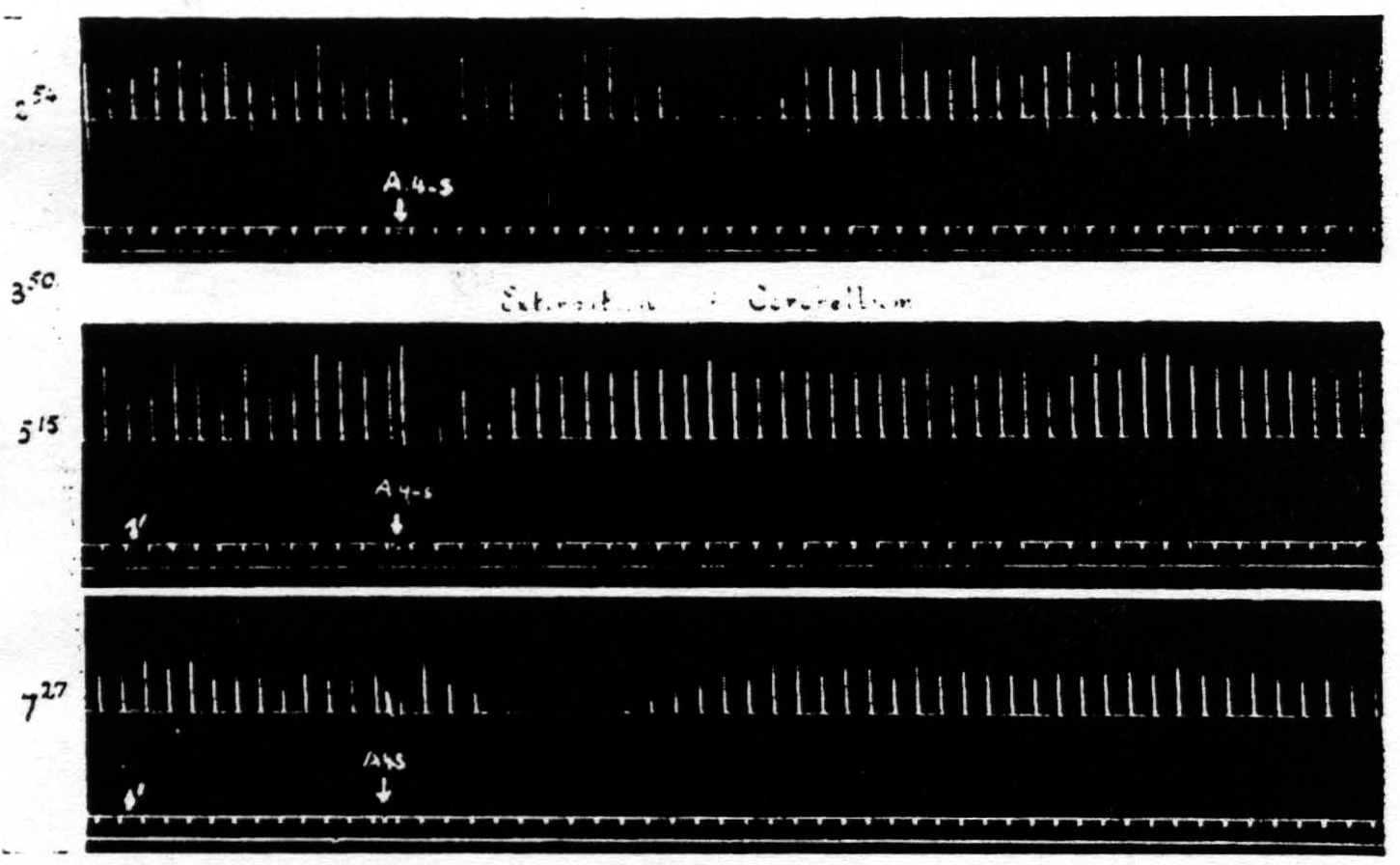
Figure 9. Feb. 22, 1939. Macaca mulatta. Dial. Record 1. Suppression of motor response elicited at 3:50 (A4 =5 sec.-0.5 μF.-50 per sec.-V.D. 2700. A4s =7.5 sec.-1 μF.-50 per sec.-V.D. 5400). Record 2. Poor suppression elicited shortly after total extirpation of cerebellum at 5:15 when threshold was high (A4 =5 sec.-0.5 μF.-50 per sec.-V.D. 4100. A4s =7.5 sec.-1 μF.-50 per sec.-V.D. 8200). Record 3. Good suppression at 7:27 when threshold had returned to original value (A4 =5 sec.-0.5 μF.-50 per sec.-V.D. 2800. A4s =7.5 sec.-1 µF.-50 per sec.-V.D. 5600).
in question. The reason for not reporting the negative cases even when they outnumbered the positive cases, is that one can deduce anything only from a positive finding. Failure to find suppression subsequent to a lesion may be due to any number of causes, but the presence of suppression after a lesion destroying some structure clearly indicates that that structure is not necessary for suppression. On looking over early records of such failures to obtain suppression it became clear that these failures usually occurred when, following the lesion, the threshold to electrical stimulation of area 4 has been raised and before it has returned to normal. Therefore, in later experiments, the suppression of motor response was sought about every hour until the
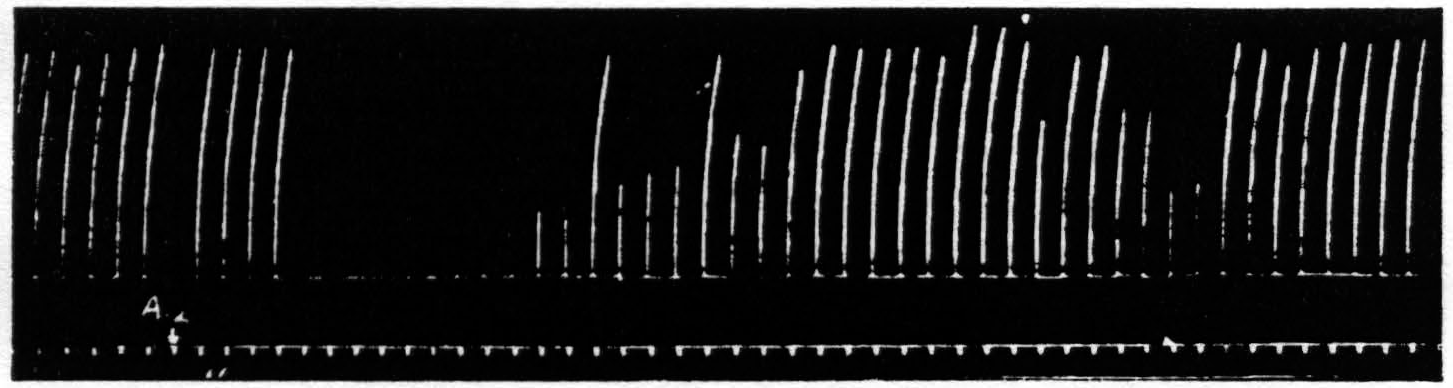
Figure 10. April 19, 1939. Macaca mulatta. Dial. Suppression of motor response to electrical stimulation (0.5 µF.-50 per sec.-V.D. 1300) of A4 produced by stimulation (1 µF.-50 per sec.-V.D. 2600) of A2. Time line =1 min.
threshold had been returned to its original value for at least one hour. This greatly reduced the number of animals that would have been required otherwise and proved that the return to approximately pre-lesion threshold was essential for suppression. (Cf. legend of Fig. 9.)
After these experiments had been completed it was discovered that stimulation in the postcentral gyrus also produced a suppression of motor response, and still more recently the same has been encountered from area 8 and from a strip of cortex lying immediately occipital to the sensory cortex. Figure 10 shows the suppression of motor response to electrical stimulation of area 4 by antecedent stimulation of the postcentral gyrus.
Discussion
The suppression of motor response to stimulation of area 4 by antecedent stimulation of area 4s has obviously raised many more questions than the investigation has been able to answer. It is well, therefore, to consider first what the experimental facts prove even if these conclusions are negative. The phenomenon itself remains interesting and can always be elicited.
First, the phenomenon has been clearly demonstrated to be something other than extinction, for the two can be separated both spatially and temporally. Second, it cannot be explained as a result of the extinction of activity in area 4 due to stimulation of 4s, for suppression of motor response occurs after a lesion of the nucleus caudatus which prevents suppression of electrical activity of area 4. Third, its long latency indicates an indirect, or complex, “delay” path. Fourth, its long duration, indicating a continuous activity of some sort (comparable to that found for after-discharge following strong cortical stimulation), together with the even longer subsequent period during which the suppression cannot be reinitiated (comparable to the duration of extinction following such an after-discharge) indicate that one is probably dealing with an activity antagonistically related to that of the motor system excited by stimulation of area 4 rather than with extinction of activity contributory to that motor system. If these suppositions be correct it follows that one is here dealing with augmented activity in a system antagonistic to the cortico-spinal system, and it becomes proper to regard suppression of motor response as an example of “inhibition,” although not in the sense in which Sherrington originally used the term to designate the relaxation of the antagonist during contraction of the reciprocally innervated agonist, since, in suppression, the relaxation involves all muscles observed. The antagonism is not between agonist and antagonist muscles but between pyramidal and extrapyramidal systems within the central nervous system and there is no evidence that activity of area 4 is antagonistic to activity of area 4s. If the antagonism is not mutual there is no reason to regard the relation between the pyramidal and extrapyramidal systems as reciprocal.
One more observation tending to the same conclusion may be cited here. In the paper concerning the motor cortex of the chimpanzee(19) it is stated that the motor after-discharge (following stimulation of areas giving motor response) was held in abeyance during stimulation of suppressor bands which have been shown to correspond to 8s, 4s, 2s and 19s in the monkey. While this finding was obtained by use of more appropriate stimulation (i.e., of longer pulse form) than was used in the experiment reported here and the work was on another species, it still seems significant to mention it here because it was obtained from the bands yielding suppression of motor response as here described, and suppression of electrical activity. Another previous finding, namely, that the deeply undercut cortex could support a prolonged after-discharge, indicating the essentially cortical nature of this type of after-discharge, shows that the holding in abeyance of the motor after-discharge can only be interpreted to mean that cortico-spinal impulses continued to be delivered to the cord but that its motor horn cells were rendered unresponsive by impulses traversing other descending systems. In this case there is no possibility of extinction; per contra, as the stimulus is there at the time and is adequate, there is every reason to expect that some system whose activity is antagonistic to cortico-spinal activity is contemporaneously active.
Thus consideration of time relations, of co-present relaxations and of holding in abeyance of after-discharge, all indicate activity in a system antagonistically related to the cortico-spinal system, i.e., “inhibition” of the response to cortico-spinal activity by contemporaneous activity of some antagonistic extrapyramidal system.
The chief difficulty with this hypothesis arises from that constancy of the knee jerk in response to weak patellar stimulation which proved that the path of the “inhibiting” disturbance was within the central nervous system. It is hard to see how the motor horn cells involved could respond normally to dorsal root stimulation and not to cortico-spinal stimulation. The difficulty may be due to insufficient knowledge of the events in the spinal cord, but until these are known the notion that suppression of motor response is a differential “inhibition” of the final common path must be held reservedly. Alternative conceptions of the site of the hypothetical “inhibition” are confronted by anatomical objections.(20)
With respect to what structures of the extrapyramidal system are involved in this suppression the seemingly most “unsuccessful” experiments are most conclusive. They have shown of one structure after another that it is not necessary to this suppression, i.e., that the remaining structures are sufficient. The only structures whose destruction has yielded a seeming “success” remain of questionable importance, namely, the ansa lenticularis, the fields of Forel and the red nucleus, and inasmuch as after the lesion was made the threshold of area 4 never returned to its original value these experiments are equivocal. What remains most surprising is that suppression of motor response does not depend upon an intact nucleus caudatus, for this has been shown to be the only part of the corpus striatum to which areas 8s and 4s send axons. Here the possibility remains that remaining parts of this structure sufficed for this suppression but not for that of electrical activity.
Summary
Motor response to electrical stimulation of area 4 is suppressed (typically, after a latency of several minutes for a duration of 5 or more minutes) by electrical or other stimulation of area 4s. It cannot be initiated again for many minutes.
This suppression is brought about by stimulation of cells in area 4s and does not require stimulation of underlying fiber tracts.
It has been shown to be distinct from extinction of motor response by temporal and spatial consideration.
It depends for its occurrence upon fiber tracts descending from 4s to deeper structures and not upon cortico-cortical connections.
It is, despite its great generality, restricted in its distribution within the central nervous system, as is proved by its failure to affect the knee-jerk.
The following structures have been shown to be severally unnecessary for its occurrence: nucleus caudatus, putamen, globus pallidus, thalamus, substantia nigra and cerebellum.
It is at present best conceived as brought about by increased activity in some portion or portions of the extrapyramidal system whose activity is antagonistically related to that of the cortico-spinal system.
Finally, it can be obtained from areas 8s, 4s, 2s and 19s, i.e., from all of those cortical areas from which suppression of electrical activity of the cortex can be obtained, although its occurrence is not dependent upon the suppression of that activity.
Footnotes
References
Dusser de Barenne, J. G. Simultaneous facilitation and extinction of motor re. sponse to stimulation of a single cortical focus. Amer. J. Physiol., 1936, 116: 39-40.
Dusser de Barenne, J. G. Physiologie der Grosshimrinde. Bumke u. Foersters Handb. Neurol., 1937, 2: 268-319.
Dusser de Barenne, J. G. Simultane Bahnung und Auslöschung in der “motorischen” Hirnrinde. Conf. neurol., 1938, 1: 2-6.
Dusser de Barenne, J. G., and McCulloch, W. S. An “ extinction” phenomenon on stimulation of the cerebral cortex. Proc. Soc. exp. Biol., N. Y., 1934, 32: 524-527.
Dusser de Barenne, J. G., and McCulloch, W. S. Functional boundaries in the sensori-motor cortex of the monkey. Proc. Soc. exp. Biol., N. Y., 1936, 35: 239-331.
Dusser de Barenne, J. G., and McCulloch, W. S. Extinction as a cortical phenomenon. Pp. 15-18 in: The jubilee symposium “The problems of nervous physiology and of behavior” dedicated to Professor I. Beritashvili (Beritoff ), Tiflis, 1936.
Dusser de Barenne, J. G., and McCulloch, W. S. Local stimulatory inactivation within the cerebral cortex, the factor for extinction. Amer. J. Physiol., 1937, 118: *510-524.
Dusser de Barenne, J. G., and McCulloch, W. S. Factors for facilitation and extinction in the central nervous system. J. Neurophysiol., 1939, 2: 319-355.
McCulloch, W. S. On the nature and distribution of factors for facilitation and extinction in the central nervous system. Amer. J. Physiol., 1937,119: 363-364.
McCulloch, W. S., and Dusser de Barenne, J. G. Extinction: local stimulatory inactivation within the motor cortex. Amer. J. Physiol., 1935, 113: 97-98.
McCulloch, W. S., and Dusser de Barenne, J. G. Action potentials of the cerebral cortex and spinal cord before and after cortical stimulation. Amer. J. Physiol., 1936, 116: 99.
Dusser de Barenne, J. G., and McCulloch, W. S. Functional organization in the sensory cortex of the monkey (Macaca mulatto). J. Neurophysiol., 1938, 1: 69-85.
Hines, Marion. The anterior border of the monkey's (Macaca mulatto) motor cortex and the production of spasticity. Amer. J. Physiol., 1936, 116: 78.
Dusser de Barenne, J. G., and McCulloch, W. S. Sensorimotor cortex, nucleus caudatus and thalamus opticus. J. Neurophysiol., 1938, 1: 364-377.
Dusser de Barenne, J. G. A method for uniform sectioning of brain. J. Tech. Methods (in press).
Hines, Marion. The “ motor” cortex. Bull. Johns Hopk. Hosp., 1937, 40: 313-336.
Levin, P. M. Efferent fibres of frontal lobe. J. comp. Neurol., 1936, 63: 369-410.
Verhaart, W. J. C., and Kennard, Margaret A. Corticofugal degeneration following thermocoagulation of areas 4, 6 and 4-s in Macaca mulatto. J. Anat., Lond., 1940, 74: 239-254.
Dusser de Barenne, J. G., Garol, H. W., and McCulloch, W. S. Motor cortex of chimpanzee. J. Neurophysiol., 1941, 4: 287-303.
Hoff, E. C. The distribution of the spinal terminals (boutons) of the pyramidal tract, determined by experimental degeneration. Proc. roy. Soc., 1932, Bill: 226-237.
For further research:
Wordcloud: Activity, After-Discharge, Antagonistic, Antecedent, Area, Barenne, Caudatus, Central, Cortex, Cortical, Dial, Dusser, Electrical, Elicited, Experiment, Extinction, Facilitation, Figure, Focus, Following, Indicate, Latency, Lesion, Macaca, McCulloch, Min, Minutes, Motor, Mulatta, Muscles, Nervous, Nucleus, Obtained, Per, Phenomenon, Prevent, Record, Remains, Response, Sec, Several, Shows, Stimulation, Structures, Suppression, System, Therefore, Threshold, Uf
Keywords: Response, Area, Cortex, Phenomenon, Stimulation, Region, Activity, Records, Line
Google Books: http://asclinks.live/ycuj
Google Scholar: http://asclinks.live/vbqk
Jstor: http://asclinks.live/jx8g