THE “MOTOR” CORTEX OF THE CHIMPANZEE12 [41]
J.G. Dusser de Barenne, Hugh W. Garol and W.S. McCulloch
Introduction
In previous papers dealing with the sensory cortex of the chimpanzee(1, 2, 3, 4) we have mentioned that in the animals upon which those experiments were performed an exploration of motor response to electrical stimulation of the cerebral cortex preceded each investigation of the location, subdivision or functional organization of the sensory cortex. These explorations differ in four respects from those reported by other investigators(5, 6, 7, 8, 9, 10) (i) The subsequent studies on the sensory cortex necessitated the use of a head holder which so fixed the jaws and immobilized the head that the tongue could never be observed and contractions of neck musculature were manifest only to palpation. (ii) The chimpanzees were under Dial narcosis of such a depth that there was usually much tension of the extensor and frequently moderate tension of the flexor muscles. (iii) The carefully controlled electrical stimulation was usually by very long pulses, often at extremely low frequencies. (iv) The area stimulated was not confined to the precentral convolution but extended over the entire exposed hemisphere, i.e., from in front of the “motor” eye field to behind the sulcus lunatus and from as far medial as could be reached with the stimulating electrodes, without displacing the brain, to the fissura Sylvii laterally. In this paper are recorded all phenomena obtained by stimulation anywhere in this extensive area.
Methods
Nine chimpanzees (Pan satyrus) 2½ to 3½ years old were used altogether. The animals were given 0.35 to 0.45 cc. Dial § per kg. body weight, ½ intraperitoneally, ½ intramuscularly. This produced full anesthesia without abolishing muscular tension. The narcotized animal was placed on a board with the hind quarters elevated to maintain good cerebral circulation and with the head fixed to the board by one bar over and behind the lower incisors and a second behind the occiput.
One hemisphere was then exposed by turning down a large osteoplastic flap. In opening the dura mater special care was taken not to injure the cerebral veins passing to the dural lacunae. Photographs from several angles were taken and printed and the location of each stimulation was recorded on these as well as on drawings of twice life-size.
In most of the experiments the contralateral arm and leg were then suspended by heavy rubber bands so disposed as to allow freedom of movement without compromising circulation. In these cases the motions were noted by two or more observers and any change in muscular tension was controlled by palpation and passive movement of the part affected.
In the earliest of these experiments an ordinary thyratron stimulator (after Schmitt(11)) was employed. Thanks to Craig Goodwin, electronic engineer for this laboratory, it was later replaced by an instrument of greater flexibility(12). This permits independent control of voltage, of number of pulses per second, of wave form, the ascending and descending phases being separately controlled, and finally, delivery of a train of impulses of any required length. When, with this new apparatus, it had been found that, except in cases of excessive narcosis or impaired cerebral blood supply, a very short ascending phase with a falling phase of from 5σ to 20σ was effective with lower voltage than sufficed with other wave forms giving comparable extinction, a simpler thyratron stimulator was constructed by Mr. Goodwin and has been employed ever since for mapping the “electrically excitable” cortex (see Fig. 1). It gives pulses at frequencies of 1 per sec. to 100 per sec. with a short ascending phase and a descending phase which can be varied from 0.5σ to 20σ. This device, like its predecessor, has an output stage designed to give practically constant voltage although the circuit through the cortex varies from 500 to many thousand ohms. With this output equal responses can be elicited at relatively constant voltage despite gross differences in the area of contact between electrodes and brain, an important consideration when so-called stigmatic electrodes are manually applied.
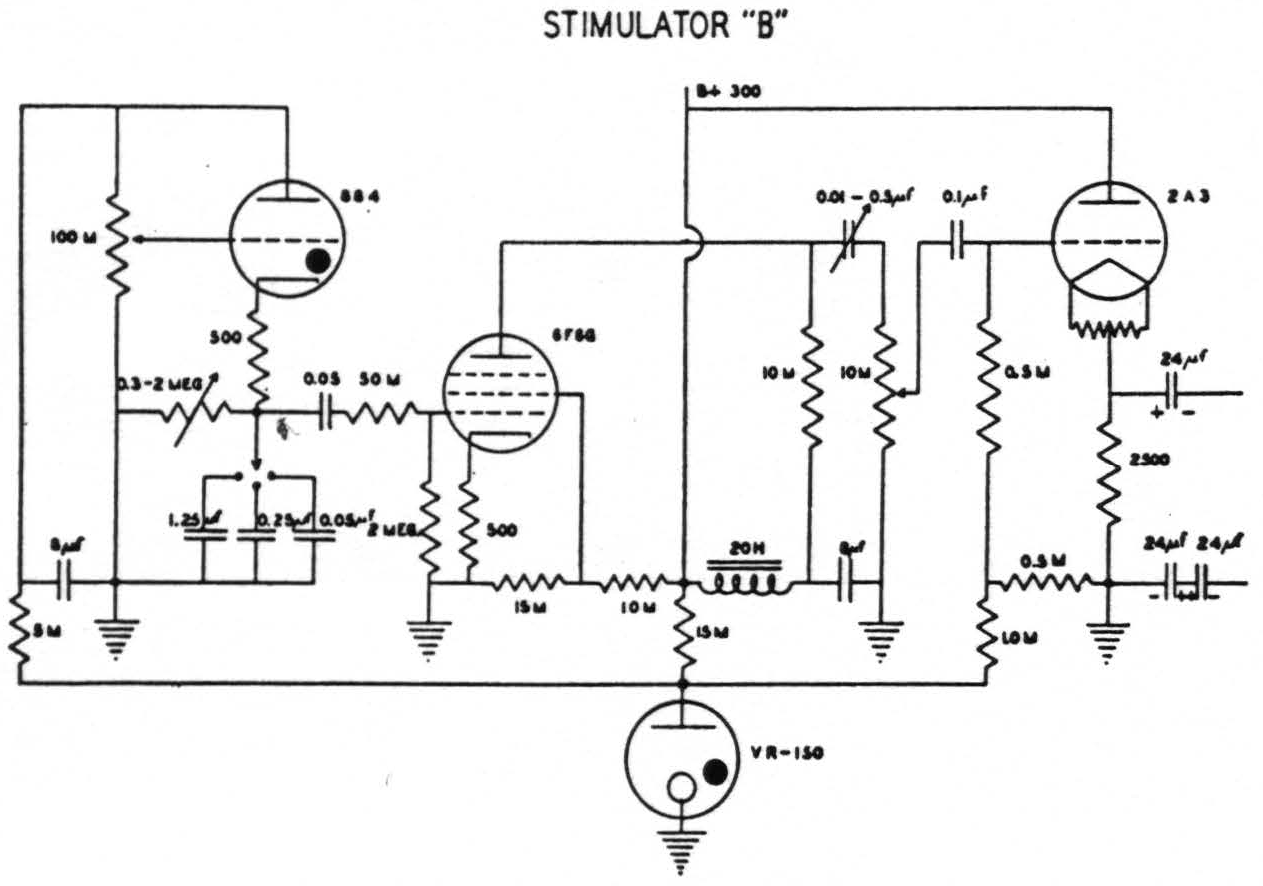
Figure 1. Circuit of stimulator “B” which gives a variable falling phase of 0.5 to 20.0σ and a frequency of 1 to 100 per second. (Designed by Craig W. Goodwin)
Stigmatic Ag-AgCl electrodes, bipolar and monopolar, were used and gave essentially similar results, except that the bipolar method with a relatively dry brain produced its motor responses at a lower voltage. Since physical considerations indicate that the bipolar method is less likely to stimulate underlying fiber tracts, quite apart from the lower voltage required, this method was generally preferred. As the voltage required for stimulation decreased until the electrodes were about 3 mm. apart and as very little change was noted when they were separated further, this distance was the one generally employed.
The frequencies used varied from 1 to 40 per sec. In general, the greater the frequency, the shorter was the duration required to elicit a motor response. In no case was stimulation prolonged enough to produce extinction,(13, 14, 15, 16, 17, 18, 19, 20) except in one experiment designed to investigate it. As a further precaution against confusing results by either extinction or facilitation, a minute or more was allowed to elapse between successive stimulations and, even then, the second stimulation was not applied in the region of the first. For experiments in which facilitation(21, 22, 23, 24, 25, 26, 27, 28, 29, 19, 30, 31) was desired, frequencies of about 30 per sec. were employed and the electrodes moved rapidly from point to point.(32) For the analysis of facilitation and extinction the wrist was fixed and the movements of the finger recorded isotonically on smoked paper and a focal cortical point for flexion of this digit was stimulated.
Although in seven of the chimpanzees both hemispheres were stimulated most of the findings reported here were obtained from the first hemisphere, for, while the second was usually in good condition when exposed, the animal had then been on the table for a matter of a day or more, the threshold had gone up and responses were less discrete. Thus only a rough map of it was warranted.
Findings
The time relations for primary facilitation (i.e., the fall in threshold, diminution of latency or increase in amplitude induced by antecedent stimulation of one and the same focus) and extinction (i.e., the rise in threshold, increase in latency or decrease in amplitude induced by antecedent stimulation of one and the same focus) and their dependence upon the parameters of stimulation are grossly similar to those found in the macaque (see Fig. 2a). The response, however, is more complicated in character and at values of stimulation near threshold the latency is much less.
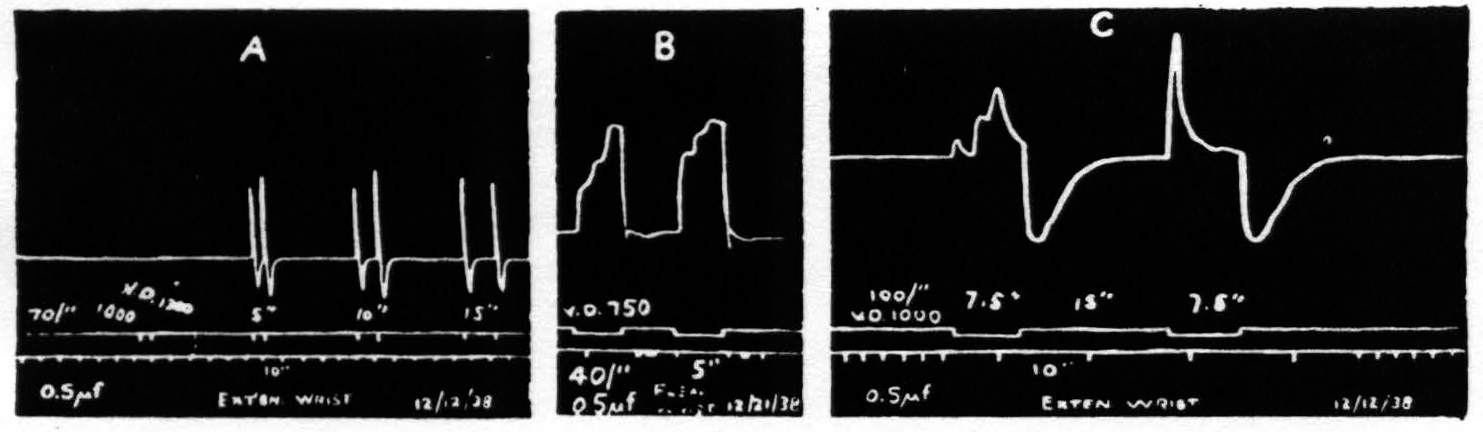
Figure. 2. Isotonic recording via air tambours. Monopolar stimulation. 0.5µf. A. Shows facilitation with intervals of 10 sec.; with partial extinction at 15 seconds. B. Shows triphasic response with more marked facilitation of the earlier components. C. Triphasic response showing facilitation of first component with extinction of subsequent components. In each case the response is followed by a contraction of the antagonistic muscles.
If the stimulation is continued for 2 to 2.5 seconds the response usually consists of 3 components. The first of these occurs, even at threshold values, with a latency too small to detect on the kymographic record. The second component appears at least a second later and the third about a second later still. With weak stimulation the second is larger than the first and the third is larger than the second. With stronger stimulation the first is disproportionately larger. No fourth component appears even when stimulation is prolonged to 7.5 sec. (see Fig. 2b). With respect to facilitation and extinction the components behave differently. As extinction is enhanced (by increasing the frequency or the duration of stimulation or by increasing the interval) the later components, particularly the third, diminish or disappear, while the first still exhibits facilitation (see Fig. 2c).
Motor bands—The lowest threshold was found immediately anterior to the fissura centralis, i.e., Band V (see Fig. 3). Immediately frontal to this band, i.e., in Band IV, the threshold was definitely higher. (Both of these regions gave highly specific movements.) Even in these regions the threshold
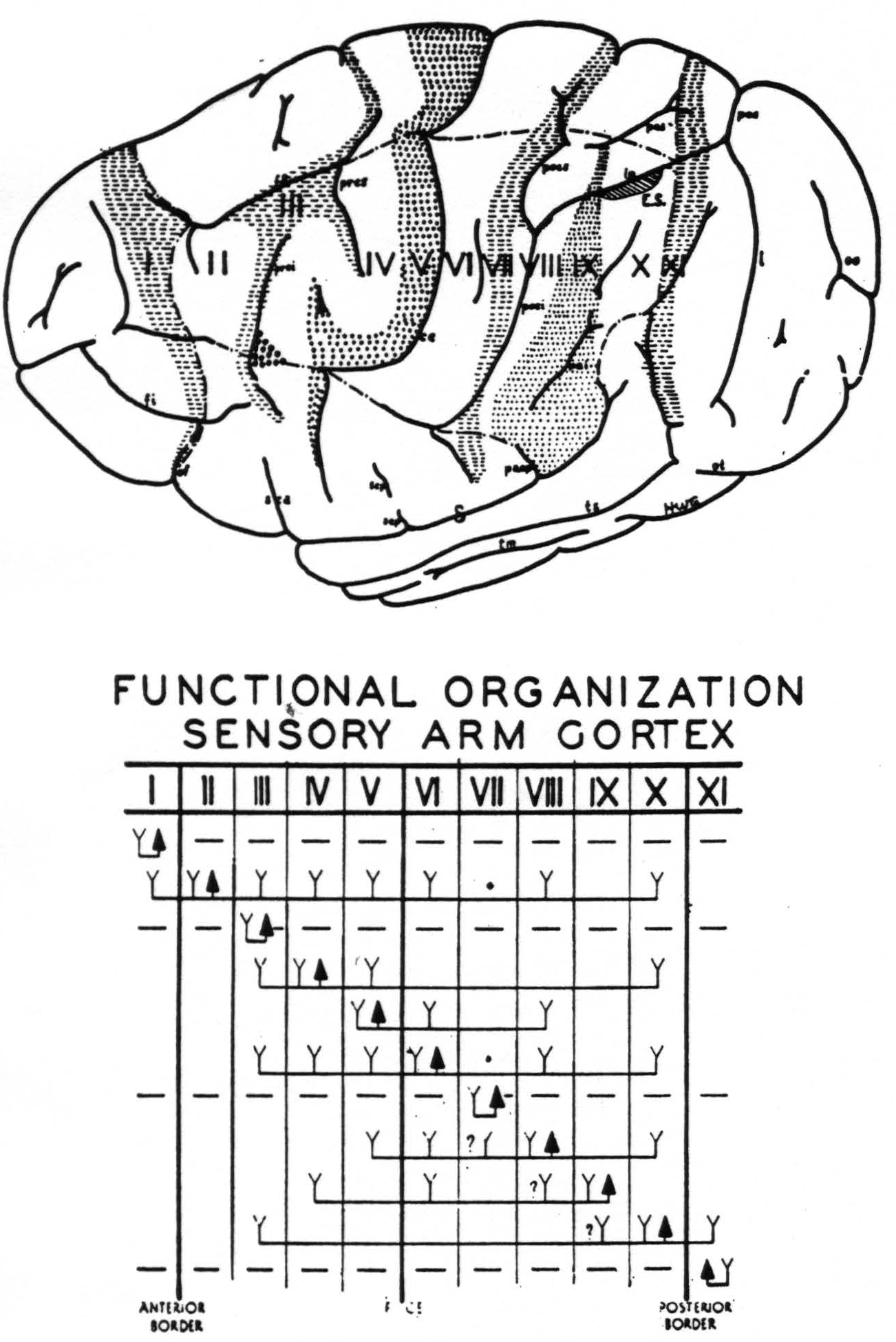
Figure 3. Above. The extent, location and functional subdivisions of the sensory arm cortex into physiologically distinguishable bands, No. II–X, and of the immediately adjacent bands, No. I and XI, indicated on a composite drawing representing the arm area in the center of the field. The bands giving suppression, No. I, III, VII and XI, are marked thus:  . Small
. Small  indicate Bands V and IX. Large
indicate Bands V and IX. Large  mark the “dud” areas. Areas between trunk and arm and between arm and neck are marked —•—•. Divisions between neck and face, and trunk and leg not marked.)
mark the “dud” areas. Areas between trunk and arm and between arm and neck are marked —•—•. Divisions between neck and face, and trunk and leg not marked.) mark the visuo-sensory band of Elliot Smith. Below. Diagrammatical representation of the directed functional (and anatomical) relations between the various cortical bands of the arm-subdivision of the sensory cortex found in these experiments and also those of Bands I and XI adjacent to but outside of the sensory cortex. Anterior and posterior borders are the limits of the sensory cortex. The suppression of the ECG of various bands upon strychninization of Bands I, III, VII and XI is indicated thus: ————. F CE = fissura centralis; * =no certain evidence; ?Y =definite “firing” but uncertainty whether strychnine invaded region so “fired.”
mark the visuo-sensory band of Elliot Smith. Below. Diagrammatical representation of the directed functional (and anatomical) relations between the various cortical bands of the arm-subdivision of the sensory cortex found in these experiments and also those of Bands I and XI adjacent to but outside of the sensory cortex. Anterior and posterior borders are the limits of the sensory cortex. The suppression of the ECG of various bands upon strychninization of Bands I, III, VII and XI is indicated thus: ————. F CE = fissura centralis; * =no certain evidence; ?Y =definite “firing” but uncertainty whether strychnine invaded region so “fired.”
was higher in the sectors for neck and truck than in those for face, arm and leg.
Band II gave mixed motor responses of leg and arm, of arm, eyes and face, and even of leg, arm and face in its most antero-medial portion. When the animal was lightly narcotized and in good condition these responses were readily elicited, whereas when it was deeply narcotized they could be obtained only after facilitation or prolonged stimulation. In the latter case they were frequently followed by motor after-discharge.
Approximately the anterior half of the postcentral convolution, Band VI, gave discrete movements which were different from those elicited from foci immediately precentral to them and that without altering the stimulus. This presumably eliminates spread of current as the efficient cause of these responses.
T. Graham Brown defines secondary facilitation: “The facilitation of a cortical point therefore seems to raise the excitability of a wide area of the surrounding cortex for the reaction evoked by the stimulation of that point.”(32, 1, 33) By secondary facilitation, starting from either the pre- or postcentral gyrus, movements were obtained from Bands VIII and IX providing there was appreciable muscular tension in the extremity involved. This was repeatedly done only in the arm region where our investigations were most extensive.
Suppressor bands(34, 35, 36, 37, 38) —Stimulation of Band I, III, VII or XI, even with 3 or 4 times the voltage which elicited response from adjacent motor foci, failed to elicit movement. Yet it had the following effects.
First, it suppressed motor response to electrical stimulation,(39, 40) i.e., after stimulation of any of these bands stimulation of a motor focus with a stimulus which previously elicited a motor response now failed to do so.
Second, it suppressed motor after-discharge following cortical stimulation, i.e., during several seconds of stimulation of any one of these bands the clonic contractions initiated by stimulation of Band II, IV, V or VI were held in abeyance. This could be repeated several times during a single motor after-discharge. More prolonged stimulation of these bands terminated it and prevented its reappearance.
Third, it relaxed existing muscular contraction, i.e., stimulation of any of these bands caused a disappearance of resistance to passive motion, a softening of muscle bellies as judged by palpation, and a falling in response to gravity of an extremity previously supported only by its muscular tension.
In order to make clear the nature of these explorations and the type of
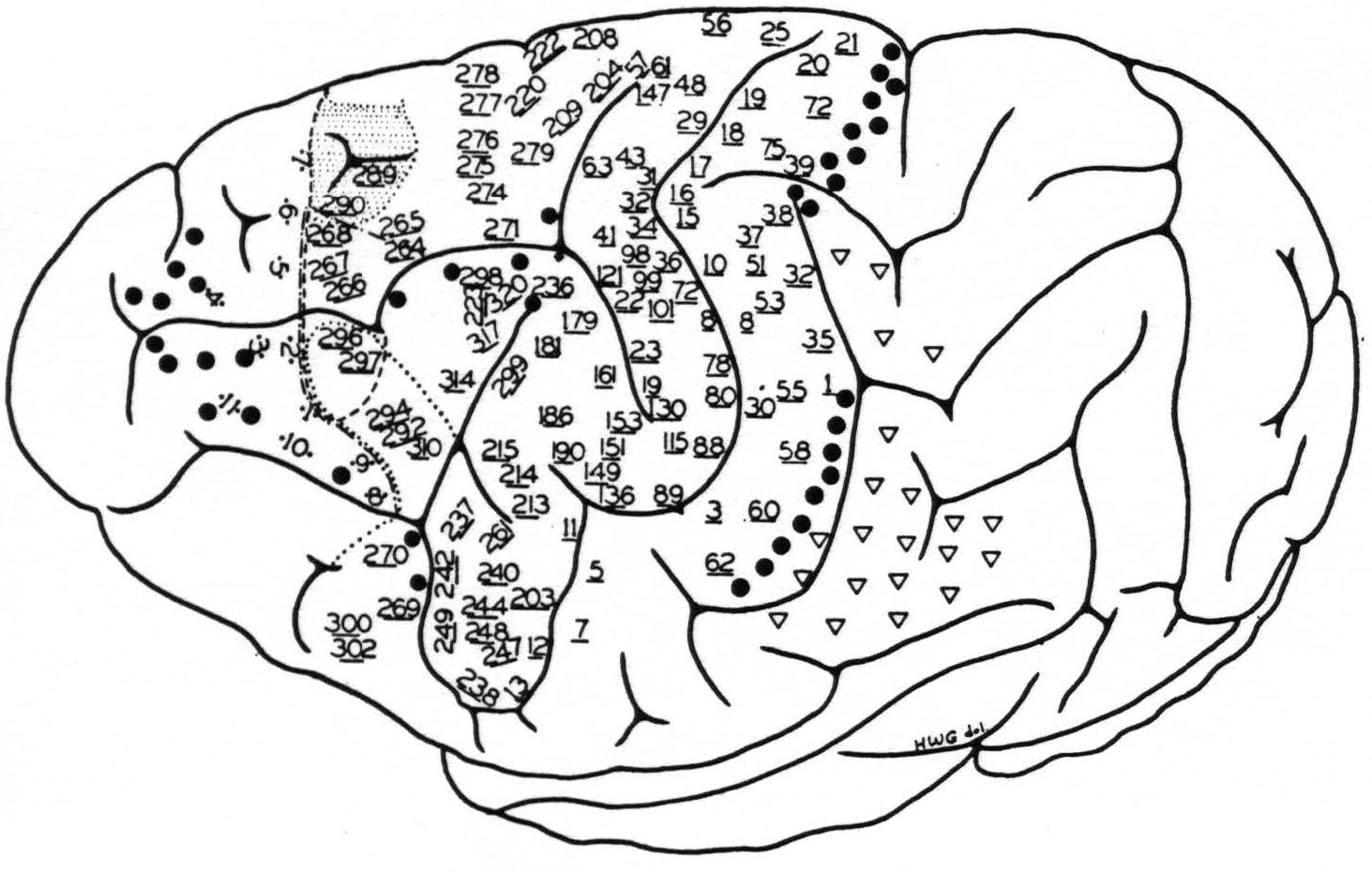
Figure 4. Chimpanzee 11. May 28, 1940.
● Electrical stimulation here elicited
1. relaxation of existing muscular tension
2. suppression of motor response
3. holding in abeyance motor after-discharge
▽ Secondary facilitation elicited motor response only when there was existing muscular “tension” in contralateral limbs.
 Stimulation here elicited simultaneous progressive movements(29, 41) of both fore and hind limbs, most marked on the contralateral side, but also some ipsilateral,(41) accompanied by alternating forceful expirations and occasionally with suggested vocalization.
--- The anterior boundary of the area eliciting motor response of the hind limb, upon facilitation.
—•—•—• The anterior boundary of the area eliciting motor response of the fore limb, upon facilitation.
•••• The anterior boundary of the area eliciting motor response of the face, upon facilitation.
* Motor point elicited contraction of the latissimus dorsi contralaterally.
The numbered points indicate sites from which primary responses were obtained. They are divided into three groups: (a) precentral, (b) postcentral and (c) eyefield. The latter points are indicated by two dots adjacent to the numbers, corresponding to the exact site and separation of electrodes. The numbers of the other two groups are underlined, indicating the site of the electrodes, their distance apart being uniformly 3 mm.
Stimulation here elicited simultaneous progressive movements(29, 41) of both fore and hind limbs, most marked on the contralateral side, but also some ipsilateral,(41) accompanied by alternating forceful expirations and occasionally with suggested vocalization.
--- The anterior boundary of the area eliciting motor response of the hind limb, upon facilitation.
—•—•—• The anterior boundary of the area eliciting motor response of the fore limb, upon facilitation.
•••• The anterior boundary of the area eliciting motor response of the face, upon facilitation.
* Motor point elicited contraction of the latissimus dorsi contralaterally.
The numbered points indicate sites from which primary responses were obtained. They are divided into three groups: (a) precentral, (b) postcentral and (c) eyefield. The latter points are indicated by two dots adjacent to the numbers, corresponding to the exact site and separation of electrodes. The numbers of the other two groups are underlined, indicating the site of the electrodes, their distance apart being uniformly 3 mm.

evidence summarized in the foregoing statements of the findings, we would cite a fair sample of the results of stimulation of the left hemisphere of chimpanzee 11, on which over 500 individual stimulations yielded discrete responses. These are given in Fig. 4 and its legend.
Discussion
A survey of previous results obtained by electrical stimulation of the cortex in any species (5, 27, 42, 41, 43, 44, 45, 46, 47, 48, 49, 50, 6, 7, 8, 9, 10, 51, 52, 53, 54, 55, 56, 57, 58, 59) discloses great variation in findings, not only from observer to observer but from observation to observation by the same observer. The work on the chimpanzee is no exception.
When one seeks the reasons for the variation he finds these fall into one of three groups related either to the animal used, the conditions of the experiment or the type of stimulation.
Most, if not all, of the electrical stimulations reported on the chimpanzee(23, 27, 6, 7, 8, 10) have been on Pan satyrus but the age and physical status of the animals have varied widely. As the group reported here ranged from 2.5 to 3.5 years and were all in good nutritional state they were to this extent alike. The configurations of the hemispheres on the other hand were so variable, even between the two hemispheres of the same animal, as to render a comparison of detailed results difficult. Fortunately, the mapping of the functional organization of the sensory cortex, which followed each stimulatory investigation, permits one to be sure in which physiologically unique band a given stimulation was performed. Without this information no such general map as Fig. 5 could be justified. Even then, however, the difficulties of fitting the findings on any particular hemisphere into the schema representing all hemispheres (those in our collection as well as those described by Mingazzini,(60) Retzius,(61) Connolly(62) and others) must be kept in mind.
Most of the electrical stimulations of the cortex of the chimpanzee have been done under ether or ether and chloroform anesthesia. In the group reported here ether was administered only long enough to render the animal manageable for administering Dial. Under its influence the threshold for electrical stimulation of the cortex remains low and relatively constant in contrast to the findings of Bucy(42, 41) and Bucy and Fulton.(43) Moreover, operations performed under Dial are not attended by vasodilatation or a rise in blood pressure commonly encountered when ether is used. While there is no generally accepted method for measuring the depth of narcosis, it may be appropriate to state that, under Dial as employed here, the electrocorticogram
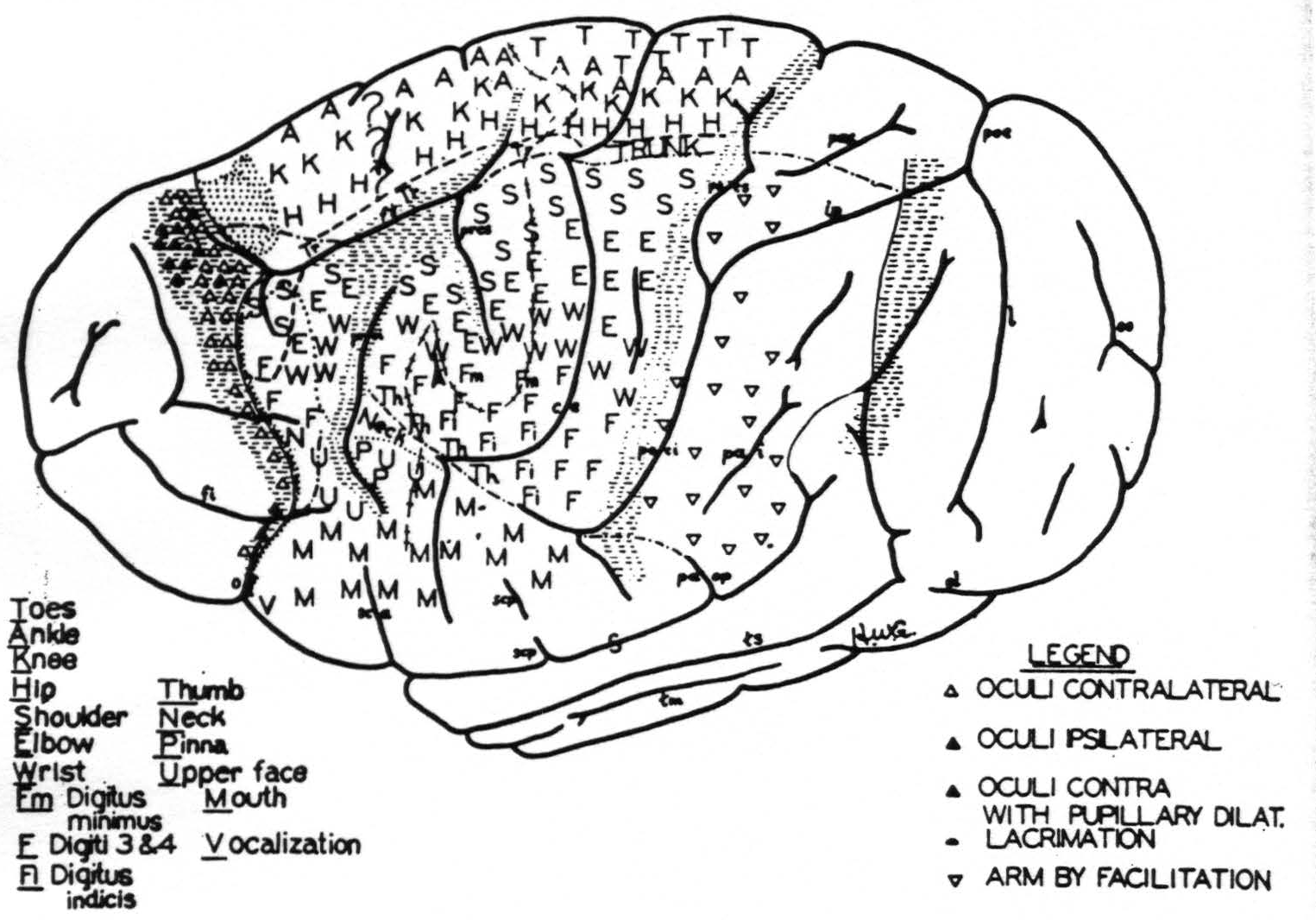
Figure 5.
was essentially similar to the electroencephalogram found in man in normal sleep,(63) but the tension of the muscles was relatively high, holding the limbs in a persistent state of semiflexion, although the corneal reflexes were absent and no responses could be elicited by nocuous stimuli.
The exposure of the brain was made with extreme care, without trauma to the brain or to the dural vessels draining it except those which had to be thermocoagulated to permit a proper field of experimentation. In all cases enough vessels remained to ensure adequate circulation. The arachnoidal membrane over the cerebral hemisphere was not ruptured and the air of the room was kept around 80°C. and of high humidity, but the surface of the arachnoid membrane was not irrigated with saline solution as is done during operations on the human brain. The cerebrospinal fluid in the subarachnoid spaces was relied upon to keep the underlying cortex moist. Neither inspissation nor any unexpected threshold changes(33, 64) were encountered in the first ten hours. Thereafter inspissation began, threshold rose and pledgets of warm Ringer's solution had to be applied at intervals in order to restore electrical excitability to about its previous level.
The fall in blood pressure under Dial(65) was compensated by elevating the foot of the animal board so that the heart was on the same level as the head.
Ag-AgCl electrodes, small spherules on the end of long pliable silver wires, were applied lightly to the cortex and that only during stimulation, thus minimizing local ischemic changes.
Type of stimulation—The first group of experiments reported here, namely those of facilitation and extinction, are sufficient to indicate how the responses obtained depend upon the parameters of stimulation. As has been indicated in previous studies on the macaque,(19) it is indeed easy to alter and even reverse the response(27) elicited from a given focus by antecedent stimulation there or elsewhere, provided it be of the right kind and at the appropriate interval. This is, in fact, sufficient to account for such phenomena as deviation of response, reversal of response, etc. It was to prevent just these alterations induced by stimulation that the type of stimulation here employed was devised for mapping the cerebral cortex. Higher frequencies, longer pulses and greater duration of stimulation are too apt to induce extinction, which disappears only after a prolonged interval, thus delaying the experiment. The higher voltage required with shorter wave forms, shorter periods of stimulation and lower frequencies inevitably increases the spread of current, and thus induces relatively complicated or diffuse movements. If time were no object one would, of course, select approximately 60-cycle stimulation to obtain even more discrete results, whereas, if the object were to obtain the maximum number of responses in a given time, he would select very short, high voltage thyratron pulses and expect diffuse results, and even those from only a small fraction of the cortex which can yield motor response upon adequate stimulation.
While everything mentioned above doubtless plays some part in determining the differences in findings, probably the biggest factor responsible for the relatively small area which yielded motor responses to the earlier investigators was the unsuspected presence of suppressor bands.(2, 35) Even when their existence is known, until they have been delimited they remain most disturbing. For when one seeks the anterior or posterior margin of a region yielding “motor” response and, by chance, stimulates a band before or behind that region he induces a suppression of motor response which, unless the muscular tension be sufficiently great for relaxation to appear as a response, is manifest only as an inability to obtain a motor response from a point which previously yielded one to the same stimulus. The works of Leyton and Sherrington and of Graham Brown suggest that accidental stimulation or spread of current to Band III prevented them from obtaining responses from the more anterior portion of Bands IV and V, and, similarly, that accidental stimulation of Band VII prevented responses from Band VI, though this may have depended partly on the wave form which they used. It is doubtful whether the experiments presented here would have been any more successful, had we not been forewarned by the results of our previous work on the suppression of motor response in the macaque.(39, 40)
That there was a significant spread of current in the experiments of Leyton and Sherrington is suggested from the size and shape of the motor eyefields, as shown in their diagram (Fig. 6). As the length of the stimulating impulse is increased and the voltage diminished, the eyefield becomes narrower.
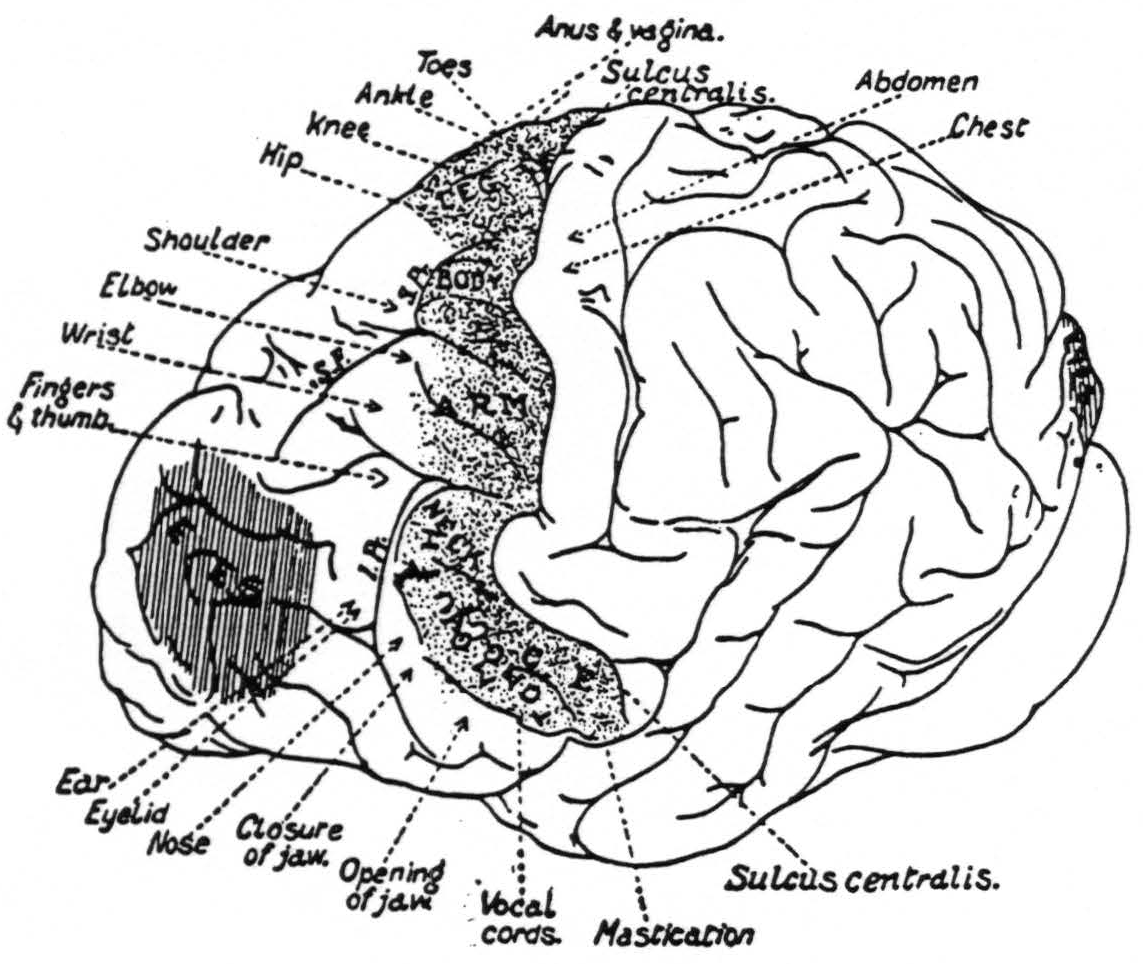
Figure 6. From Grünbaum and Sherrington.
For example, when one traces point to point by “secondary facilitation” from the sulcus centralis, anteriorly or posteriorly, the suppressor bands interfere with the procedure, for if, in so doing, these bands are stimulated, no further responses are obtainable. One has, therefore, first to delimit these suppressor bands so that he can omit stimulation within them and resume it beyond them. A typical example for the anterior boundaries of leg, arm and face area is given in Fig. 4, as is also an indication of the far postcentral regions from which one can obtain motor responses by secondary facilitation, starting from foci in the postcentral gyrus which yield primary motor responses. It should be noted, however, that the response elicited from these far postcentral regions depends on the presence of muscular tension in the animal, a condition which is prevented by stimulation of Band VII or any other suppressor band. This is of especial interest for, while the nature and distribution of the factors for facilitation and extinction are fairly well known, neither the mode nor the site of operation of suppression of motor responses is yet established. Yet it would be as premature to attempt to conjecture what part this loss of tension may play in suppression of motor response as it would to attempt to explain how stimulation of the suppressor bands can hold in abeyance motor after-discharge following cortical stimulation. The outstanding fact which will have to be taken into consideration, in forming any hypothesis as to suppression of motor response, is this: it is obtainable from exactly the same areas as those which yield a suppression of electrical activity of the cerebral cortex.(2)
A comparison of the sensory cortex of the chimpanzee (Fig. 5) with that of the macaque(34) ^(see fig. 1)^ reveals an essential similarity of functional organization which not even the great discrepancy in size, shape and functional differentiation of the hemisphere can conceal.
It was hoped that in the work on the chimpanzee finer differentiations of function could be distinguished, as, indeed, was sometimes possible. However, the results with respect to Band I were in the main disappointing, for, while it was possible to find separate foci for a large number of discrete eye movements, in every hemisphere these occurred among foci for suppression, whether judged by relaxation of muscular tension, suppression of motor response or holding in abeyance an after-discharge.
Band II, lying immediately behind Band I, shows strikingly similar properties in both its “motor” and its “sensory” functions. It will be recalled that strychninization of any subdivision of this, the most anterior band of the sensory cortex, produced disturbances which were not confined to the subdivision of the cortex strychninized and in this same band, though primary movements restricted to some part of the extremity subserved by the subdivision in question could be elicited, experiments involving facilitation showed a wide overlapping seen nowhere else in the sensory cortex. The anterior boundary of Band II is fixed and easily found because of the suppression elicited from the band immediately in front of it. The same is true of its posterior boundary throughout the arm and part of the face regions, but in the leg region the posterior boundary is more difficult to locate, lying anterior to the ascending ramus of the superior precentral sulcus.
If one regards the superior precentral and the adjacent superior frontal sulci as comparable to the superior precentral in the macaque, and the ascending ramus of the inferior precentral as a homologue of the spur of the arcuate, Band III obviously represents area 4s, the “strip” in the macaque.(38) This should indicate a cytoarchitectonic division between these areas which Brodmann numbered 4 and 6 and which have been called respectively the “motor” and “premotor” regions.(66, 67)
In this case area 4 is divided into two bands, IV and V. They are dissimilar as regards both functional organization of the sensory cortex and threshold to electrical stimulation, a difference well known in the macaque (Hines). It is interesting in this connection to note that for cytoarchitectonic reasons von Economo(68, 69) divides Brodmann's(70) area 4 into two bands, FA and FAγ, which may correspond to our Bands IV and V in the chimpanzee.
The electrical excitability of the immediate postcentral band, VI, is entirely in harmony with the findings reported in the literature concerning monkeys,(14, 45, 56) orang,(5) and man(46, 47, 48, 9) and enough has been said of the discrepant findings in the chimpanzee. Its posterior margin conforms curiously closely to that found in man. That margin is bounded by the suppressor region, Band VII, which has its functional homologue in the brain of the macaque (area 2s). While no previous investigators have reported responses to stimulation posterior thereto the conditions have never been the same.
That responses could be elicited by electrical stimulation of the posterior parietal sensory cortex, Bands VIII and IX, was, in view of statements to the contrary in the literature, most surprising. The only definite indication that movements could be elicited from this region in the monkey is given in the drawing of C. and O. Vogt(71) (see p. 438) and from this region in man cited by Foerster.(46, 47, 48)
The failure to obtain ocular movements from this same region seems, in view of the findings in the macaque, to indicate that the stimulus here employed did act upon the cells of this region rather than upon some subjacent fiber system and, therefore, tends to strengthen rather than to weaken the importance of our findings of motor response from this region.
The posterior margin of this “excitable” or “motor” posterior parietal region may well be defined by the suppressor band, XI, which lies just outside the sensory cortex.
This suppressor band, XI, which has its counterpart in the macaque" lies in a region whose position and shape suggests area 19 of Brodmann. With respect to results of electrical stimulation it resembles the three suppressor bands previously described.
The major conclusion of these investigations on the chimpanzee, covering both the functional organization of the sensory cortex and the “motor” response to electrical stimulation of the same hemispheres, is that “motor” responses can be elicited by appropriate electrical stimulation from practically all parts of the cerebral cortex which, by the criteria used, can be considered “sensory,” except those bands, III and VII, where stimulation produced muscular relaxation instead of contraction.
Three considerations militate against the simple statement that this “sensory cortex” is also “the motor cortex.” First, there is the factual difficulty with respect to the suppressor bands, III and VII, for while a relaxation is as truly a response as is a contraction, inclusion of bands giving relaxation would bring under this caption Bands I and XI, neither of which is “sensory” and one of which at least includes points motor for the eyes. Second, responses to electrical stimulation of the parietal regions depend, as shown in the experiments here cited, upon two adjuvant factors, namely, great tension in the muscles to be moved and facilitation of the secondary type beginning with the electrical stimulation of a point giving a primary response. The third consideration is a matter of terminology. For historical reasons the term “motor” cortex now means primarily “area 4,” whereas the term “sensory” cortex has come to mean that region strychninization of which produces, in the non-narcotized state, symptoms of somatic sensory excitation.(72, 73) Had the term “sensory” cortex been coined to designate that part of the cortex in which most discrete somatotopic localization is found,(74, 20) it would have had a much more restricted meaning, implying the postcentral region, par excellence. For all these reasons it seems best to state the conclusions as follows.
Conclusions
Appropriate electrical stimulation of the cerebral cortex of the chimpanzee under moderate Dial narcosis exhibits facilitation and extincton of motor response. Although their relation within a single response is more complex than in the macaque their dependence upon the parameters of stimulation is the same.
Stimulation with parameters selected to avoid facilitation and extinction, or to obtain facilitation when necessary to elicit responses from regions otherwise unresponsive, reveal the following:
With the exception of two narrow bands practically all parts of the cortex which, by the criterion here used, can be considered sensory yield contraction of skeletal muscles.
These two bands, III and VII, within (and two others, I and XI, just without) the sensory cortex yield (i) suppression of motor response to cortical stimulation, (ii) suppression of motor after-discharge following cortical stimulation and (iii) relaxation of skeletal muscles.
With the exception of the parietal area, which requires facilitation and existing muscular tension to exhibit motor response to cortical stimulation, the bands of the cortex revealed by electrical stimulation and motor response are identical with the bands revealed by strychninization and recording of electrical activity.
Footnotes
References
Bailey, P., Dusser de Barenne, J. G., Garol, H. W., and McCulloch, W. S. Sensory cortex of the chimpanzee. Amer. J. Physiol., 1940, 129: 303-304.
Bailey, P., Dusser de Barenne, J. G., Garol, H. W., and McCulloch, W. S. Sensory cortex of chimpanzee. J. Neurophysiol., 1940, 3: 469-485.
Dusser de Barenne, J. G. The sensory cortex of the chimpanzee's brain. Science, 1939, 90: 403-404.
Dusser de Barenne, J. G., and McCulloch, W. S. The sensory cortex of the chimpanzee. Proc. Soc. exp. Biol., N. Y., 1939, 42: 27-29.
Beevor, C. E., and Horsley, V. A record of the results obtained by electrical excitation of the so-called motor cortex and internal capsule in an orang-outang (Simia satyrus). Philos. Trans., 1890, 181B: 129-158.
Grünbaum, A. S. F., and Sherrington, C. S. Observations on the physiology of the cerebral cortex of some of the higher apes. Proc. roy. Soc., 1901, 69B: 206-209.
Grünbaum, A. S. F., and Sherrington, C. S. Observations on the physiology of the cerebral cortex of the anthropoid apes. *Thompson, Yates and Johnston Lab. Report, 1903, 5: 55-58.
Grünbaum, A. S. F., and Sherrington, C. S. Observations on the physiology of the cerebral cortex of the anthropoid apes. Proc. roy. Soc., 1903-04, 72:152-155.
Horsley, V. The function of the so-called motor area of the brain. (Linacre Lecture) Brit. med. J., 1909, 2: 125-132.
Leyton, A. S. F., and Sherrington, C. S. Observations on the excitable cortex of the chimpanzee, orang-utan and gorilla. Quart. J. exp. Physiol., 1917, 11: 135-222.
Schmitt, O. H. A., and Schmitt, F. O. A universal precision stimulator. Science, 1932, 76: 328-330.
Goodwin, C. W., and McCulloch, W. S. Apparatus for cortical stimulation with separately variable ascending and descending phases. (Unpublished)
Dusser de Barenne, J. G. Simultaneous facilitation and extinction of motor response to stimulation of a single cortical focus. Amer. J. Physiol., 1936, 116: 39-40.
Dusser de Barenne, J. G. Physiologie der Grosshimrinde. Bumke u. Foersters Handb. Neurol., 1937, 2: 268-319.
Dusser de Barenne, J. G. Simultane Bahnung und Ausloschung in der “motorischen” Himrinde. Conf. Neurol., 1938, 1: 1-4.
Dusser de Barenne, J. G., and McCulloch, W. S. An “extinction” phenomenon on stimulation of the cerebral cortex. Proc. Soc. exp. Biol., N. Y., 1934, 32: 524-527.
Dusser de Barenne, J. G., and McCulloch, W. S. Extinction: local stimulatory inactivation within the motor cortex. Amer. J. Physiol., 1935, 113: 97.
Dusser de Barenne, J. G., and McCulloch, W. S. Local stimulatory inactivation within the cerebral cortex, the factor for extinction. Amer.J. Physiol., 1937,118:510-524.
Dusser de Barenne, J. G., and McCulloch, W. S. Factors for facilitation and extinction in the central nervous system. J. Neurophysiol., 1939, 2: 319-355.
Marshall, W. H., Woolsey, C. N., and Bard, P. Representation of tactile sensibility in the cerebral cortex of the monkey as indicated by cortical potentials, pp. 168-173 in: MacLeod's Physiology in Modern Medicine, 1938, 8th ed.
Brown, T. Graham. The phenomenon of augmentation of excitability in the motor cortex. J. Physiol., 1914, 48: 29-30.
Brown, T. Graham. Motor activation of the postcentral gyrus. J. Physiol., 1914, 48: 30-31.
Brown, T. Graham. Note on the functions of the postcentral gyrus in the anthrapoid ape. J. Physiol., 1914, 48: 33.
Brown, T. Graham. Studies in the physiology of the nervous system. XXII: On the phenomenon of facilitation. 1: Its occurrence in reactions induced by stimulation of the “motor” cortex of the cerebrum in monkeys. Quart. J. exp. Physiol., 1915, 9: 81-99.
Brown, T. Graham. Studies in the physiology of the nervous system. XXIII: On the phenomenon of facilitation. 2: Its occurrence in response to subliminal cortical stimuli in monkeys. Quart. J. exp. Physiol., 1915, 9: 101-116.
Brown, T. Graham. Studies in the physiology of the nervous system. XXVI: On the phenomenon of facilitation. 5: Additional note on “secondary facilitation” in the cortical mechanism in monkeys. Quart. J. exp. Physiol., 1916, 10: 97-144.
Brown, T. Graham, and Sherrington, C. S. On the instability of a cortical point. Proc. roy. Soc., 1912, 85B: 250-277.
Brown, T. Graham, and Sherrington, C. S. Note on the functions of the cortex cerebri. J. Physiol., 1913, 46: 22.
Bubnoff, N., and Heidenhain, R. Ueber Erregungs- und Hemmungsvorgänge innerhalb der motorischen Hirncentren. Pflüg. Arch. ges. Physiol., 1881, 26: 137-200.
Exner, S. Zur Kenntnis von der Wechselwirkung der Erregungen im Zentralnervensystem. Pflüg. Arch. ges. Physiol., 1882, 28: 487-506.
Lorente de Nó, R. Facilitation of motoneurones. Amer. J. Physiol., 1935, 113: 505-523.
Brown, T. Graham. Studies in the physiology of the nervous system. XXIV: On the phenomenon of facilitation. 3: “Secondary facilitation” and its location in the cortical mechanism itself in monkeys. Quart. J. exp. Physiol., 1915, 9:117-130.
Clark, R. H. Investigations of the central nervous system. (Johns Hopk. Reports) Baltimore, Johns Hopkins Press, 1920, 47 pl., 165 pp.
Dusser de Barenne, J. G., Garol, H. W., and McCulloch, W. S. Functional organization of sensory and adjacent cortex in the monkey. J. Neurophysiol., 1941, 4:304-310.
Dusser de Barenne, J. G., and McCulloch, W. S. Functional organization in the sensory cortex of the monkey (Macaco mulatto). J. Neurophysiol., 1938, 1: 69-85.
Dusser de Barenne, J. G., McCulloch, W. S., and Ogawa, T. Functional organization in the face-subdivision of the sensory cortex of the monkey (Macaco mulatta). J. Neurophysiol., 1938, 1: 436-441.
Garol, H. W. Some observations on the suppression of electrical activity of areas 4 and 6. Amer. J. Physiol., 1940,129:361.
Hines, Marion. The “motor” cortex. Johns Hopk. Hosp. Bull., 1937, 60: 313-336.
Dusser de Barenne, J. G., and McCulloch, W. S. Suppression of motor response upon stimulation of area 4-s of the cerebral cortex. Amer. J. Physiol., 1939, 126: P482.
Dusser de Barenne, J. G., and McCulloch, W. S. Suppression of motor response from area 4 by stimulation of area 4s. J. Neurophysiol., 1941, 4: 311-323.
Bucy, P. C. A comparative cytoarchitectonic study of the motor and premotor areas in the primate cortex. J. comp. Neurol., 1935, 62: 293-332.
Bucy, P. C. Electrical excitability and cytoarchitecture of the premotor cortex in monkeys. Arch. Neurol. Psychiat., Chicago, 1933, 30: 1205-1225.
Bucy, P. C., and Fulton, J. F. Ipsilateral representation in the motor and premotor cortex of monkeys. Brain, 1933, 56: 318-342.
Cushing, H. A note upon the faradic stimulation of the postcentral gyrus in conscious patients. Brain, 1909-10, 32: 44-53.
Ferrier, D. The functions of the brain. London, Smith Elder and Co., 1876, xv, 323 pp.
Foerster, O. The cerebral cortex in man. Lancet, 1931, 221: 309-312.
Foerster, O. The motor cortex in man in the light of Hughlings Jackson's doctrines. (The Ninth Hughlings Jackson Lecture) Brain, 1936, 59: 135-159.
Foerster, O., and Penfield, W. Der Narbenzug am und im Gehirn bei traumatischer Epilepsie in seiner Bedeutung für das Zustandekommen der Anfälle und für die therapeutische Bekämpfung derselben. Z. ges. Neurol. Psychiat., 1930, 125: 475-572.
Franz, S. I. Variations in distribution of the motor centers. Psychol. Monogr., 1915, 19: 80-162.
Fritsch, G., and Hitzig, E. Ueber die elektrische Erregbarkeit des Grosshirns. Arch. Anat. Physiol., Lpz., 1870, 37: 300-332.
Mills, C. K. Physiological areas and centres of the cerebral cortex of man, with new diagrammatic schemes. Penna. Univ. Med. Bull., 1904-05, 17: 90-98.
Penfield, W., and Boldrey, E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain, 1937, 60 : 389-443.
Penfield, W., and Gage, L. Cerebral localization of epileptic manifestations. Arch. Neurol. Psychiat., Chicago, 1933, 30: 709-727.
Rioch, D. McK., and Rosenblueth, A. Inhibition from the cerebral cortex. Amer. J. Physiol., 1935, 113: P110; 663-676.
Roaf, H. E., and Sherrington, C. S. Experiments in examination of the “locked-jaw” induced by tetanus toxin. J. Physiol., 1906, 34: 313-331.
Rothmann, M. Ueber die elektrische Erregbarkeit der Zentralwindungen. Mschr. Psychiat. Neurol., 1912, 32: 489-502.
Scarff, J. E. Primary cortical centers for movements of upper and lower limbs in man: Observations based on electrical stimulation. *Arch. Neurol. Psychiat., Chicago, 1940, 44: 243-299.
Vogt, C., and Vogt, O. Zur Kenntnis der elektrisch erregbaren Hirnrindengebiete bei den Säugetieren. J. Psychol. Neurol., Lpz., 1906-07, 8: 277-456.
Ward, J. W., and Clark, S. L. Specific responses elicitable from subdivisions of the motor cortex of the cerebrum of the cat. J. comp. Neurol., 1935-36, 63: 49-64.
Mingazzini, G. Beitrag zur Morphologie der äusseren Grosshimhemisphärenober-fläche bei den Anthropoiden (Schimpanse und Orang). Arch. Psychiat. Nervenkr., 1928, 85: 1-219.
Retzius, G. Das Affenhim in bildlicher Darstellung. Jena, etc., G. Fischer, 1906, 67 pl., 22 pp.
Connolly, C. J. Fissural pattern of the primate brain. Amer. J. Physical Anthropol., 1936, 21: 301-422.
Davis, H., Davis, P. A., Loomis, A. L., Harvey, E. N., and Hobart, G. Human brain potentials during the onset of sleep. J. Neurophysiol., 1938, 1: 24-38.
Gotsch, F., and Horsley, V. On the mammalian nervous system, its functions, and their localisation determined by an electrical method. (Croonian Lecture) Philos. Trans., 1891, 182B: 267-526.
Fulton, J. F., Liddell, E. G. T., and Rioch, D. McK. “Dial” as a surgical anaesthetic for neurological operations; with observations on the nature of its action. J. Pharmacol., 1930, 40: 423-432.
Fulton, J. F. A note on the definition of the “motor” and “premotor” areas. Brain, 1935, 58: 311-316.
Walshe, F. M. R. On the “syndrome of the premotor cortex” (Fulton) and the definition of the terms “premotor” and “motor”: with a consideration of Jackson's views on the cortical representation of movements. Brain, 1935, 58: 49-80.
Von Economo, C. The cytoarchitectonics of the human cerebral cortex. Oxford University Press, 1929, xii, 186 pp.
Von Economo, C., and Koskinas, G. N. Die Cytoarchitektonic der Hirnrinde der erwachsenen Menschen. Wien u. Berlin, J. Springer, 1925, xiv, 810 pp.
Brodmann, K. Vergleichen de Lokalisationslehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues. Leipzig, J. A. Barth, 1909, xii, 324 pp.
Vogt, C., and Vogt, O. Allgemeinere Ergebnisse unserer Himforschung. J. Psychol. Neurol., Lpz., 1919, 25: 277-462.
Dusser de Barenne, J. G. Experimental researches on sensory localization in the cerebral cortex of the monkey (Macacus). Proc. roy. Soc., 1924, 96B: 272-291.
Dusser de Barenne, J. G. Experimentelle Untersuchungen über die Lokalisation des sensiblen Rindengebietes im Grosshim des Affen (Macacus). *Dtsch. Z. Nervenheilk., 1924, 83: 273-299.
Bard, P. Studies on the cortical representation of somatic sensibility. (Harvey Lecture) Bull. N. Y. Acad. Med., 1938, 14: 585-607.
Campbell, A. W. Histological studies on the localization of cerebral functions. Cambridge University Press, 1905, xi, 360 pp.
Holmes, G., and May, W. P. On the exact origin of the pyramidal tracts in man and other mammals. Brain, 1909-10, 32: 1-43.
Levin, P. M., and Bradford, F. K. The exact origin of the cortico-spinal tract in the monkey. J. comp. Neurol., 1937-38, 68: 411-422.
McCulloch, W. S. On the nature and distribution of factors for facilitation and extinction in the central nervous system. Amer. J. Physiol., 1937, 119: 363-364.
Sharpey-Schafer, E. A. Textbook of physiology. Edinburgh, etc., Y. J. Pentland, 1900, 2 vol.
Smith, G. Eliot. A new topographical survey of the human cerebral cortex, being an account of the distribution of the anatomically distinct cortical areas and their relationship to the cerebral sulci. J. Anat. Physiol., 1907, 41: 237-254.
For further research:
Wordcloud: Animal, Anterior, Area, Arm, Bands, Barenne, Brain, Brown, Cerebral, Chimpanzee, Cortex, Cortical, Dusser, Electrical, Elicited, Experiments, Extinction, Facilitation, Fig, Findings, Functional, Graham, Hemisphere, Indicate, Macaque, McCulloch, Monkey, Motor, Movements, Muscular, Neurol, Observations, Obtained, Physiol, Physiology, Point, Postcentral, Region, Response, Results, Sensory, Stimulation, Suppression, Suppressor, Tension, Threshold, Voltage, Xi
Keywords: Chimpanzee, Response, Cortex, Stimulation, Papers, Studies, Area, Hemisphere, Motor, Location
Google Books: http://asclinks.live/9d4z
Google Scholar: http://asclinks.live/gqq2
Jstor: http://asclinks.live/dnj7