PHYSIOLOGICAL DELIMITATION OF NEURONES IN THE CENTRAL NERVOUS SYSTEM12 [32]
J.G. Dusser de Barenne and W.S. McCulloch
##
Heretofore for the delimitation of neurons in the central nervous system (CNS) the only methods available have been anatomical: the Marchi method, the Weigert method and the method of retrograde cell degeneration. Although fully recognizing the admirable services these methods have rendered, one should not be blind to their limitations. These methods are time-consuming and require in experimentation sterile operative procedures with a survival of the animal for weeks or even months. These are only small practical advantages; heavier weigh the theoretical objections to these methods. We have not in mind here so much the uncertainty often present in the diagnosis of a positive Marchi degeneration, but rather two other objections: 1, the Marchi method does not and can not reveal the actual site of ending of the degenerating myelinated axons, for the last millimeters of these have no myelin sheaths; 2, this method cannot help out in the case of non-myelinated neurons. Both objections hold also for the Weigert method, which, furthermore, because it provides a “negative” picture of the degeneration, permits a diagnosis only if the degeneration has occurred in a fairly great number of axons gathered in more or less compact bundles. Similarly the diagnosis of retrograde cell degeneration can only be made if the cells which have atrophied or disappeared were numerous and formed a fairly compact nuclear mass.
For the last four years we have used in this laboratory a combination of physiological methods which has shown itself admirably suitable for the study of problems of “functional organization” in the CNS (1, 2, 3, 4, 5, 6, 7), namely, the local strychninization of a mass of gray matter in combination with recording of the electrical activity of some other gray matter in the CNS. Strychnine solution applied to a few square millimeters of superficial gray matter, i.e., local strychninization of this mass, results in a typical change in its electrical activity, namely, the appearance of large, rapid voltage fluctuations, “strychnine-spikes.” When the local strychninization has been performed somewhere in the sensorimotor cortex these spikes do not remain restricted to the small area strychninized but are also found in other specific areas of this cortex. The distribution of the strychnine-spikes is specific for each strychninized area. The observations that local strychninization of an area a (for instance area 6) produces spikes in the electrogram of some other area b (for instance area 4), whereas local strychninization of area b does not produce spikes in the electrogram of area a show that this combination of methods reveals directed functional relations and, therefore, directed anatomical relations. To correlate these findings with the neuronal structure of the cortex two suppositions are possible: 1, that strychnine acts on the axonal terminations and produces its remote effects by antidromic “firing” of the cell bodies of the neurons whose axonal endings are strychninized; 2, that strychnine acts on the cell bodies (and [or] the synapses incrusting these) and produces its remote effects in the direction of the normal conduction in the neurons. That the first of these suppositions is false and the second true follows from the following observations. Local strychninization of the dorsal horn of a spinal segment invariably evokes symptoms of sensory excitation (paresthesiae, hyperesthesia, hyperalgesia) in the corresponding “strychnine-segment zone” and only in this zone (8). The axonal terminations converging upon the perikarya of the posterior horns of a spinal segment are the endings of neurons originating in many other spinal segments and supraspinal levels. If the strychninization of these axonal terminations “fired” antidromically the cell bodies of the neurons to which they belong, it is impossible to conceive how the sensory symptoms could be projected by the animal into the periphery, into the strychnine-segment zone of the locally strychninized spinal segment and in this zone only. On this supposition the symptoms of sensory excitation should be absent altogether or present in a much larger area of the body, namely, in all portions subserved sensorially by the neurons “fired” antidromically. A second observation which precludes the first supposition is that local strychninization of the posterior horn of a spinal segment does not produce strychnine-spikes in the corresponding posterior root (unpublished experiments). The only alternative left is, therefore, the second supposition mentioned, namely, that the strychnine acts on the cell bodies (and [or] the synapses incrusting them) and produces its remote effects in the direction of the normal, i.e., forward, conduction in the reflex arc.
This view is strengthened by the following observations. Local strychninization of a specific area of the sensorimotor cortex, to wit, areas L. or A.4-s, produces strychnine-spikes in the electrogram of the nucleus caudatus, whereas strychninization of this nucleus does not “fire” the cortex (6). Several neuroanatomists are inclined to admit the existence of cortico-caudate neurons, all deny the existence of caudato-cortical neurons.
Local strychninization of the sensorimotor cortex “fires” the sensory thalamic nuclei and local strychninization of these nuclei “fires” the sensorimotor cortex. Here, neuroanatomy recognizes both cortico-thalamic and thalamo-cortical neurons. All available evidence, therefore, points towards the thesis that strychnine acts on the perikarya (and [or] the synaptic endings incrusting them), causes these structures to discharge impulses and, by axonal propagation of these impulses, produces strychnine-spikes in those remote, related, portions of gray matter where axons originating in strychninized nerve cells or collaterals of such axons end. If this thesis is accepted, local strychninization with recording of the electrical activity in the course of an acute experiment of a few minutes in the living (narcotized) animal gives the origin and termination of neurons. Thus one obtains physiological delimitation of neurons in the CNS.
This thesis has been verified again in a new series of experiments on the sensory systems between the nuclei gracilis (Goll) and cuneatus (Burdach) and the sensory cortex. For a clear understanding it is necessary to say a few words about the make-up of these systems. In the nuclei gracilis and cuneatus originate the fibers of the medial fillet (lemniscus medialis) which end in the sensory nuclei of the optic thalamus, where begin the tertiary sensory neurons that end in the sensory cortex. Present-day anatomy asserts that all fibers of the medial fillet have a synaptic interruption in the thalamus; a few of the older neuroanatomists admitted also the existence of an uninterrupted path from the nuclei gracilis and cuneatus to the sensory cortex, i.e., of a direct cortical fillet. In the descending direction, while neuroanatomy recognizes the existence of cortico-thalamic neurons, there is nothing to suggest the existence of neurons descending from the sensory cortex to the nuclei gracilis and cuneatus.
If there is no such descending system and if there is a direct cortical fillet, one obviously has an opportunity for verification of our thesis. For then strychninization of the arm and leg regions of the sensory cortex should not produce strychnine-spikes in the electrogram of the nuclei gracilis and cuneatus, whereas strychninization of these nuclei should result in strychnine-spikes in the electrocorticograms of the leg and arm subdivisions of the sensory cortex.
When put to the test in experimentation both predictions are verified. Even strychninization of the cortex of almost the entire postcentral gyrus (leg and arm) produces no change in the electrograms of the Goll and Burdach nuclei; strychninization of these nuclei produces definite strychnine-spikes in the electrograms of the corresponding subdivisions (leg and arm) of the sensorimotor cortex.
In explanation of the appearance of these strychnine-spikes two possibilities present themselves: 1, that these spikes result from “firing” of cells in the nuclei gracilis and cuneatus whose axons end directly in the sensory cortex; 2, that they are the expression of disturbances propagated to the cortex through synaptic relays in the thalamus. Transsynaptic conduction of strychnine-spikes under the conditions of this experiment is improbable for reasons which will be discussed later. But quite apart from the possibility of transsynaptic conduction of the strychnine-spikes, the problem at hand can be settled by removing the thalamus without injury to the internal capsule—through which the neurons of the direct cortical fillet, if present, must ascend—and then repeating the experiment.
Therefore, in a monkey (macaca mulatta) under Dial-narcosis, one hemisphere was completely removed and the thalamus of the remaining hemisphere scooped out. Then a pair of stigmatic electrodes was placed on the sensory cortex in area arm 4 (A.4). Local strychninization of the contralateral nucleus cuneatus resulted in the appearance of definite strychnine-spikes in the electrocorticogram of A.4.
This cortex showed, naturally, very little spontaneous electrical activity due to the extirpation of its thalamus, by which procedure the cortico-thalamo-cortical circuits, necessary for the maintenance of the normal electrocorticogram, were destroyed. In this experiment the nucleus cuncatus and the arm region of the sensory cortex were preferred to the nucleus gracilis and the leg region because the sensory arm nuclei of the thalamus lie medial to the leg nuclei (9); thus total extirpation of the arm nuclei without injury to the internal capsule can be performed with greater certainty.
Records 2 and 3 of figure 1 show the presence of strychnine-spikes in the electrogram of the sensory (arm) cortex 17 and 25 minutes respectively after strychninization of the contralateral nucleus cuneatus; record 4 shows that these spikes have disappeared again 35 minutes after this strychninization. Figure 2 shows the brain from which these records were obtained. This experiment demonstrates that local strychninization of the nucleus cuneatus still produces strychnine-spikes in the sensory arm cortex, even in the acute experiment, after extirpation of the thalamus.
In two other similar experiments no such spikes appeared and the autopsies revealed that in these monkeys not only was the thalamus removed, but in one case the posterior limb of the internal capsule interrupted, and in the other the medial fillet just below the level of the thalamus. From this evidence it follows that in the nucleus cuneatus originate neurons, whose axons run through the internal capsule directly to the sensory (arm) cortex. In other words, there exists a direct cortical fillet.
It almost goes without saying that when the thalamus is left intact, strychninization of the nuclei gracilis and cuneatus produces strychnine-spikes of the electrograms of the corresponding sensory thalamic nuclei (leg and arm nuclei), thus revealing the existence of neurons originating in the nuclei gracilis and cuneatus and terminating in the thalamus (secondary sensory neurons). The existence of the tertiary neurons of this sensory system and their distribution in the sensory cortex has already been established, as mentioned above, by these physiological methods in previous experiments (5).
In these experiments on the relations of the nuclei gracilis and cuneatus and the thalamic nuclei, three electrodes, insulated except for their tips, intended for “triangulation” of the electrical activity of the thalamus were usually introduced into it. That is, the three electrodes (a, b and c) were
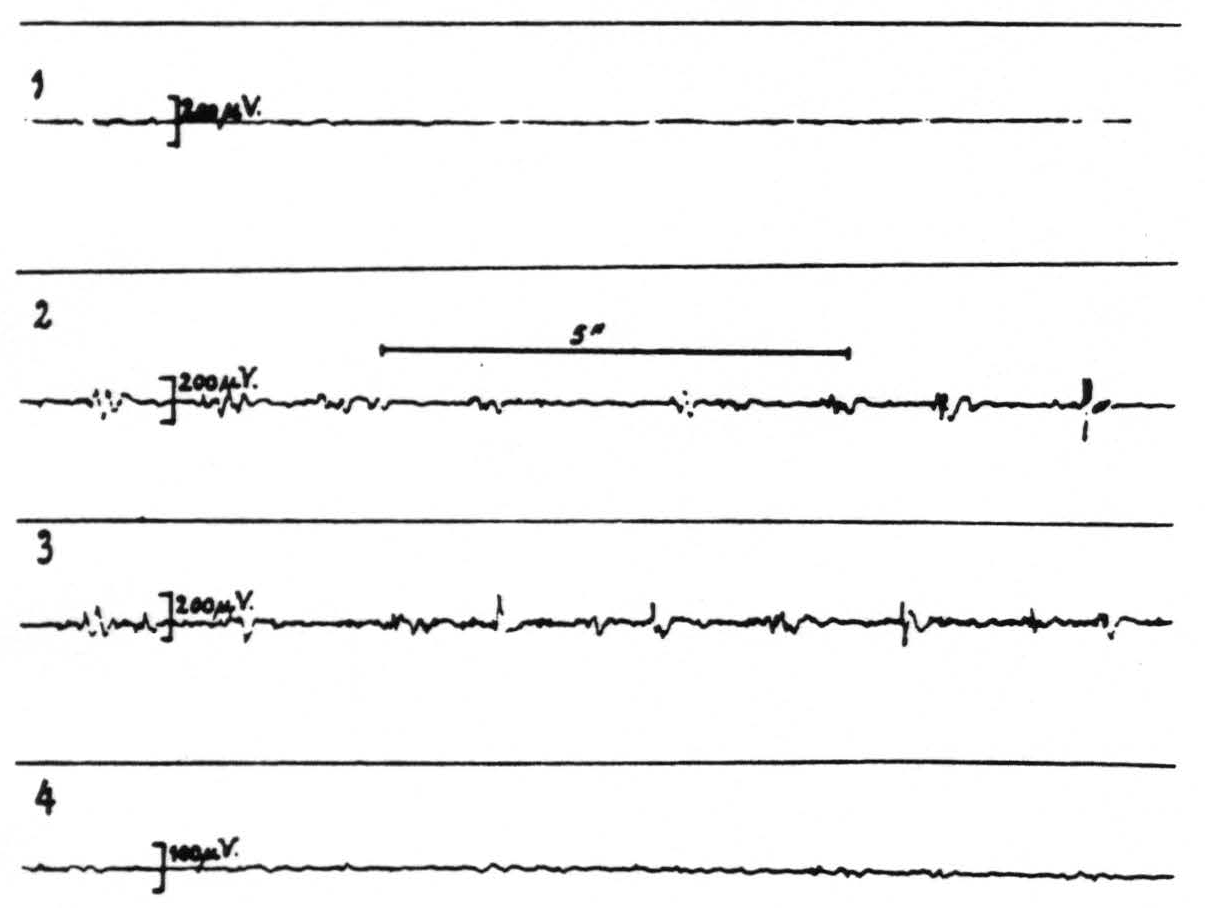
Figure 1. Macaca mulatta. Dial-narcosis. Total extirpation of the left hemisphere and scooping out of the right thalamus. Four electrograms from the right precentral arm cortex (A.4) before (record 1) and after (records 2, 3 and 4) local strychninization of the left nucleus cuneatus. Record 2 taken 17 minutes after this strychninization, record 3, 8 minutes later, record 4 again 10 minutes later (35 minutes after the strychninization) when strychnine-spikes in records 2 and 3 have disappeared. Note that the electrical activity of the cortex is very small, because of the interruption of the cortico-thalamo-cortical circuits by the extirpation of the thalamus.
linked up with three oscillographs in the following manner: electrodes a and b to oscillograph 1, electrodes b and c to oscillograph 2 and electrodes c and a to oscillograph 3. This arrangement gives perhaps in the simplest way clear-cut evidence where a disturbance evidenced in the electrograms originates. If originating at or in the neighborhood of electrode a it will appear in oscillograms 1 and 3; if the disturbance starts at or near electrode b it will manifest itself in oscillograms 1 and 2; if the disturbance originates at or near electrode c it will appear in oscillograms 2 and 3.
In one of these experiments strychnine-spikes appeared in the electrograms referable to electrodes a and c, but not in those to electrode b. See figure 3.
At autopsy it was found that, because of forgetting in this particular experiment to orient the head in space, the electrodes were not introduced into the thalamus but that electrodes a and c were in the globus pallidus whereas b was in the putamen!
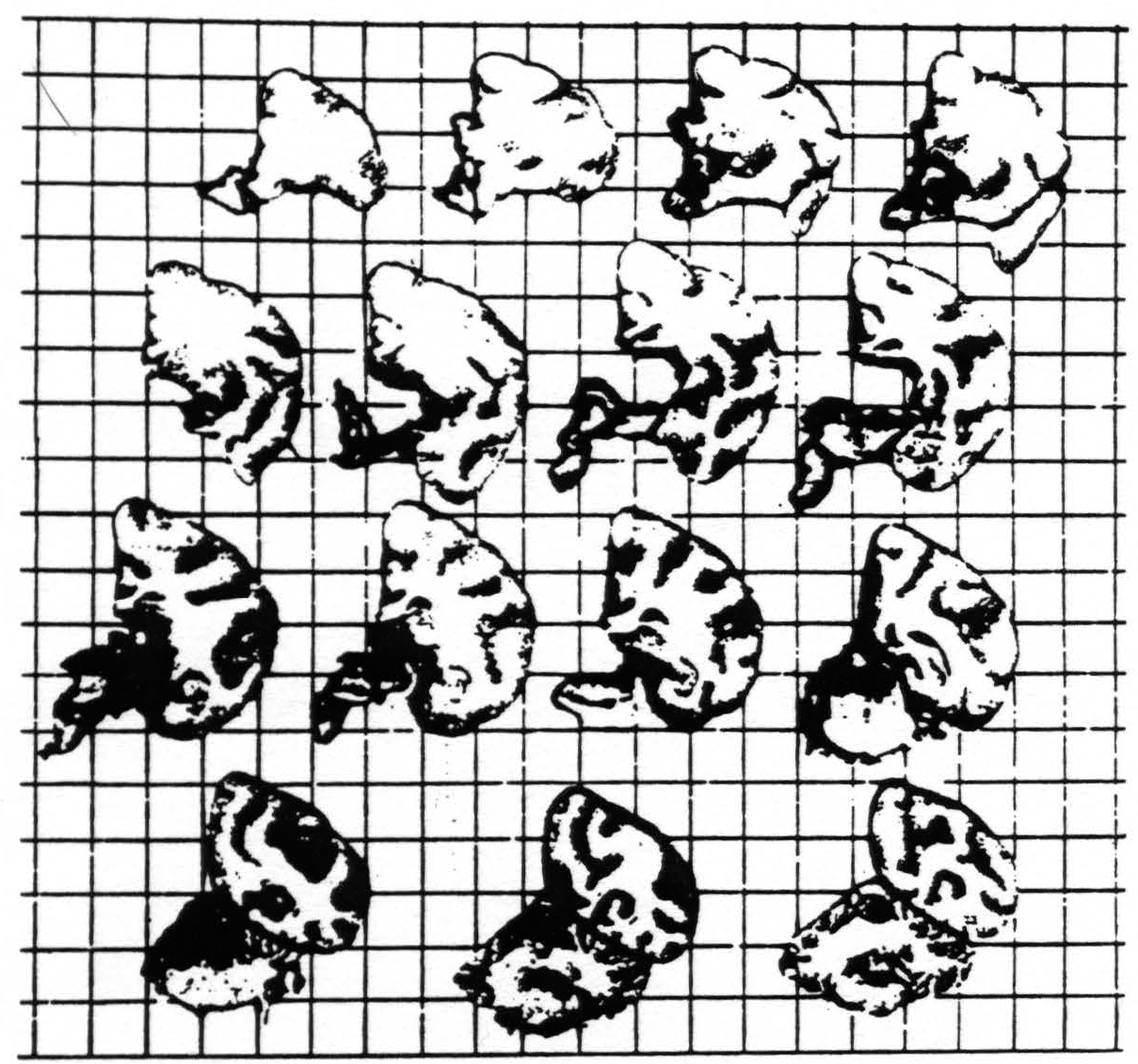
Fig. 2 shows brain of experiment of figure 1. Total extirpation of left hemisphere and right thalamus. Squares of background represent square centimeters.
In records II and III of figure 3 it will be seen that strychnine-spikes are present simultaneously in electrograms 2 and 3 and in 1 and 3 respectively, indicating that leads a and c (which were in the pallidum) were “fired.” The absence of a simultaneous disturbance in electrograms 1 and 2 denotes that electrode c, lying in the putamen, was not fired.
Electrode a was so close to the fibers of the internal capsule, although definitely in the gray matter of the pallidum, that it might be thought that the spikes referable to this electrode might be spikes “passing by” in the fibers of the internal capsule, namely, spikes in the fibers of the direct cortical fillet fired by the strychninization of the nuclei gracilis and cuneatus.
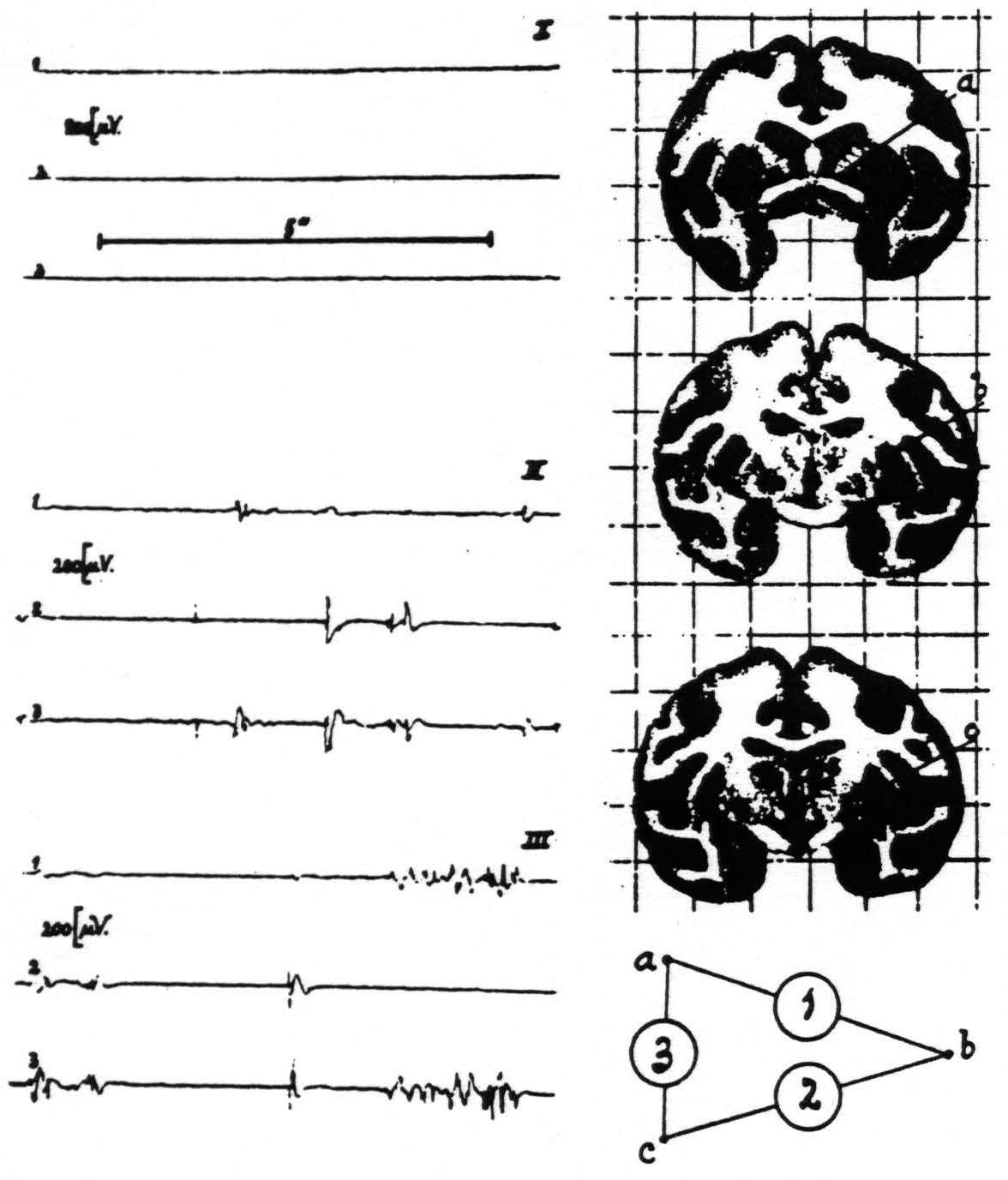
Figure 3. Macaca mulatta. Dial-narcosis. Electrograms taken by the “triangulation” arrangement (see text) from pallidum and putamen. Position of electrodes and their “hook-up” at right of records. Record I (control) before, records II and III after local strychninization of contralateral nuclei of Goll and Burdach. Simultaneous disturbances in electrograms 1 and 3 denote “firing” at electrode a (in pallidum), such in electrograms 2 and 3 firing at electrode c (in pallidum). Absence of synchronous disturbances in electrograms 1 and 2 signifies absence of firing at electrode b (in putamen).
This possibility cannot be accepted, however, for electrode c, whose tip lay in the peripheral portion of the inner segment of the globus pallidus and it will be seen in figure 3 that the most typical strychnine-spikes appear in electrograms 2 and 3, referable to electrode c.
We were much surprised by this firing of the pallidum by the strychninization of the nuclei gracilis and cuneatus, indicating the existence of neurons originating in these nuclei and terminating in the pallidum, for we did not know of the existence of such neurons. But scrutiny of the literature revealed that Bechterew as far back as 1885 (see his Leitungs-bahnen im Gehirn and Rückenmark, [A. Georgi, Leipzig], 1899, pp. 239-241) on the basis of myelogerietic studies indicated the existence of such a pathway and that Cajal in his Histologie du système nerveux ([Maloine, Paris], vol. 2, 1911 p., 511) describes collaterals arising from fibers of the medial fillet and ending in the globus pallidus.
One point remains for discussion, namely, the question of transsynaptic propagation of the strychnine-spikes. This whole series of experiments on strychninization of the nuclei gracilis and cuneatus and recording the electrical activity of the sensory cortex was started to investigate this very problem on the assumption that all secondary sensory neurons originating in these nuclei terminate in the thalamus. The unexpected physiological demonstration of neurons from these nuclei running directly up to the sensory cortex precluded the intended investigation in this particular system. All that can be said at present is that so long as one adheres to the procedure of local strychninization in the narcotized animal there is no direct evidence of such transsynaptic propagation. By local strychninization is meant either the strychninization of only a few square millimeters of superficial gray matter of the CNS (cortex, spinal cord, etc.) or the injection of a minute quantity of a strychnine solution (less than 1 cubic millimeter of a 1 to 3 per cent solution) into deep structures of the CNS (thalamus and other subcortical structures).
Such local strychninization when performed on the “motor” cortex of the (narcotized) macaque never results in twitches in the peripheral striped musculature. These only appear when the strychninization is much more extensive or often repeated without waiting for the effect of previous local strychninizations to wear off. Obviously such twitches must be the expression of transsynaptic disturbances, though it is still an open question whether these are synchronized enough to resemble typical strychnine-spikes.
Occasionally, after strychninizations which are not strictly local, one observes in the electrogram of gray matter of the CNS, which according to present-day neuroanatomy is only in transsynaptic connection with the strychninized gray, a small and relatively slow “bump”. This, however, is not easily confused with the typical strychnine-spike.
Until evidence to the contrary is forthcoming we feel compelled to regard the appearance of strychnine-spikes in the electrogram of a gray mass of the CNS following local strychninization of another gray mass as evidence of the strychninization of neurons originating in the mass strychninized and ending in or giving collaterals to the gray matter where the spikes appear. Therefore, this combination of physiological methods allows one in the course of an acute experiment of a few minutes’ duration to delimit the origin and ending of neurons in the CNS, and thus to confirm anatomy where it is known, to settle mooted anatomical questions and even to discover unknown neuronal connections which have not been and cannot be investigated adequately by anatomical methods now known.
Conclusions
It is shown that the method of local strychninization of gray matter combined with the recording of the electrical activity of another mass of gray matter of the CNS permits one in the course of an acute experiment of a few minutes in the fully narcotized animal to decide whether in the strychninized gray mass originate neurons ending, directly or with axonal collaterals, in the gray matter whose electrical activity is recorded. If strychnine-spikes appear in this electrogram the answer is in the affirmative, if no spikes appear in the negative.
With these physiological methods the existence of a direct cortical fillet (from the nuclei cuneatus and gracilis to the sensory cortex) and the existence of neurons originating in the nuclei cuneatus and gracilis and ending in the globus pallidus has been demonstrated.
This combination of physiological methods, therefore, permits the delimitation of neurons in the central nervous system.
Footnotes
References
Dusser de Barenne, J. G. and W. S. McCulloch. Proc. Soc. exper. Biol. and Med. 35: 329, 1936.
Dusser de Barenne, J. G. and W. S. McCulloch. Trans. Am. Neurol. Assn. 171, 1936.
Dusser de Barenne, J. G. This Journal 119: 263, 1937.
Dusser de Barenne, J. G. and W. S. McCulloch. J. Neurophysiol. 1: 69, 1938.
Dusser de Barenne, J. G. and W. S. McCulloch. J. Neurophysiol. 1: 176, 1938.
Dusser de Barenne, J. G. and W. S. McCulloch. J. Neurophysiol. 1: 364, 1938.
Dusser de Barenne, J. G., W. S. McCulloch and T. Ogawa. J. Neurophysiol., 1: 436, 1938.
Dusser dr Barenne, J. G. Folia Neurobiologica, 76: 277, 1912.
Dusser de Barenne, J. G. and O. Sager. Arch. Neurol. and Psychiat. Chicago, 38: 913, 1937.
For further research:
Wordcloud: Activity, Appear, Area, Arm, Axons, Barenne, Cell, Cortex, Cuneatus, Direct, Disturbance, Dusser, Electrical, Electrode, Electrograms, Ending, Existence, Experiment, Figure, Fillet, Firing, Gracilis, Gray, Leg, Local, Mass, Matter, McCulloch, Methods, Minutes, Neurons, Nuclei, Nucleus, Originating, Pallidum, Physiological, Present, Produces, Records, Segment, Sensory, Spikes, Spinal, Strychnine-Spikes, Strychninization, System, Terminations, Thalamus
Keywords: Methods, Degeneration, Neurons, Method, Systems, Axons, Millimeters, Imagination, Advantages, Objections
Google Books: http://asclinks.live/2mu8
Google Scholar: http://asclinks.live/g845
Jstor: http://asclinks.live/7tqj