WHAT THE FROG'S EYE TELLS THE FROG'S BRAIN 12 [146]
J.Y. Lettvin, H.R. Maturana, W.S. McCulloch and W.H. Pitts
Summary
In this paper, we analyze the activity of single fibers in the optic nerve of a frog. Our method is to find what sort of stimulus causes the largest activity in one nerve fiber and then what is the exciting aspect of that stimulus such that variations in everything else cause little change in the response. It has been known for the past 20 years that each fiber is connected not to a few rods and cones in the retina but to very many over a fair area. Our results show that for the most part within that area, it is not the light intensity itself but rather the pattern of local variation of Intensity that is the exciting factor. There are four types of fibers, each type concerned with a different sort of pattern. Each type is uniformly distributed over the whole retina of the frog. Thus, there are four distinct parallel distributed channels whereby the frog's eye informs his brain about the visual image in terms of local pattern independent of average illumination. We describe the patterns and show the functional and anatomical separation of the channels. This work has been done on the frog, and our interpretation applies only to the frog.
Introduction
Behavior of a Frog
A frog hunts on land by vision. He escapes enemies mainly by seeing them. His eyes do not move, as do ours, to follow prey, attend suspicious events, or search for things of interest. If his body changes its position with respect to gravity or the whole visual world is rotated about him, then he shows compensatory eye movements. These movements enter his hunting and evading habits only, e.g., as he sits on a rocking lily pad. Thus his eyes are actively stabilized. He has no fovea, or region of greatest acuity in vision, upon which he must center a part of the image. He also has only a single visual system, retina to colliculus, not a double one such as ours where the retina sends fibers not only to colliculus but to the lateral geniculate body which relays to cerebral cortex. Thus, we chose to work on the frog because of the uniformity of his retina, the normal lack of eye and head movements except for those which stabilize the retinal image, and the relative simplicity of the connection of his eye to his brain.
The frog does not seem to see or, at any rate, is not concerned with the detail of stationary parts of the world around him. He will starve to death surrounded by food if it is not moving. His choice of food is determined only by size and movement. He will leap to capture any object the size of an insect or worm, providing it moves like one. He can be fooled easily not only by a bit of dangled meat but by any moving small object. His sex life is conducted by sound and touch. His choice of paths in escaping enemies does not seem to be governed by anything more devious than leaping to where it is darker. Since he is equally at home in water and on land, why should it matter where he lights after jumping or what particular direction he takes? He does remember a moving thing providing it stays within his field of vision and he is not distracted.
Anatomy of Frog Visual Apparatus
The retina of a frog is shown in Fig. 1(a). Between the rods and cones of the retina and the ganglion cells, whose axons form the optic nerve, lies a layer of connecting neurons (bipolars, horizontals, and amacrines). In the frog there are about 1 million receptors, 2½ to 3½ million connecting neurons, and half a million ganglion cells.(1) The connections are such that there is a synaptic path from a rod or cone to a great many ganglion cells, and a ganglion cell receives paths from a great many thousand receptors. Clearly, such an arrangement would not allow for good resolution were the retina meant to map an image in terms of light intensity point by point into a distribution of excitement in the optic nerve.
There is only one layer of ganglion cells in the frog. These cells are half a million in number (as against one million rods and cones). The neurons are packed together tightly in a sheet at the level of the cell bodies. Their dendrites, which may extend laterally from 50µ to 500µ interlace widely into what is called the inner plexiform layer, which is a close-packed neuropil containing the terminal arbors of those neurons that lie between receptors and ganglion cells. Thus, the amount of overlap of adjacent ganglion cells is enormous in respect to what they see. Morphologically, there are several types of these cells that are as distinct in their dendritic patterns as different species of trees, from which we infer that they work in different ways. The anatomy shown in the figures is that found in standard references. Further discussion of anatomical questions and additional original work on them will appear in a later publication.
Physiology as Known up to This Study
Hartline(2) first used the term receptive field for the region of retina within which a local change of brightness would cause the ganglion cell he was observing to discharge. Such a region is sometimes surrounded by an annulus, within which changes of brightness affect the cell's response to what is occurring in the receptive field, although the cell does not discharge to any event occurring in the occurring in the annulus alone. Like Kuffier,(4) we consider the receptive field and its interacting annulus as a single entity, with apologies to Dr. Hartline for the slight change in meaning. Hartline found three sorts of receptive field in the frog: ON, ON-OFF, and OFF. If a small spot of light suddenly appears in the receptive field of an ON-cell, the discharge soon begins, increases in rate to some limit determined by the intensity and area of the spot, and thereafter slowly declines. Turning off the spot abolishes the discharge.
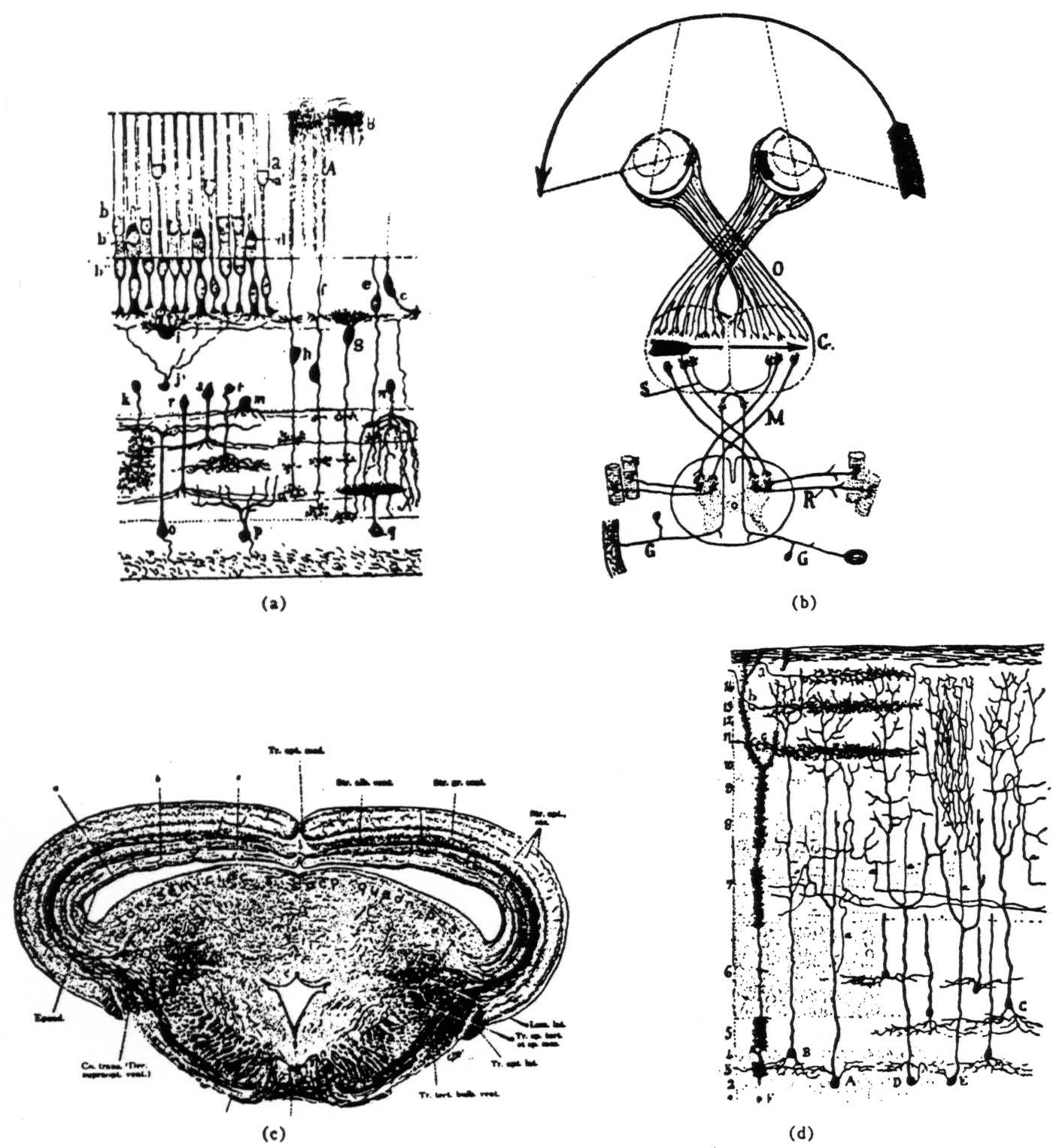
Figure 1. (a) This ia a diagram of the frog retina done by Ramon y Cajal over 50 years ago.(3) The rods and cones are the group of elements in the upper left quarter of the picture. To their bushy bottom ends are connected the bipolar cells of the intermediate layer, for example., f, g, and h. Lateral connecting neurons, called horizontal and amacrine cells, also occur in this layer, for example. i,j and m. The bipolars send their axons down to arborize in the inner plexiform layer, roughly the region bounded by cell m above and the bodies of the ganglion cells, o, p and q, below. In this sketch, Ramon has the axons of the bipolar cells emitting bushes at all levels in the plexiform layer; in fact, many of them branch at only one or two levels. Compare the dendrites of the different ganglion cells. Not only do they spread out at different levels in the plexiform layer, but the patterns of branching are different. Other ganglion cells, not shown here, have multiple arbors spreading out like a plane tree at two or three levels. If the terminals of the bipolar cells are systematically arranged in depth, making a laminar operational map of the rods and cones in terms of very local contrast, color, ON, OFF, etc., then the different shapes of the ganglion cells would correspond to different combinations of the local operations done by the bipolars. Thus would arise the more complex operations of the ganglion cells as described in the text, (b) This is Ramon y Cajal's diagram of the total decussation or crossing of the optic nerve fibers in the frog.(3) He made this picture to explain the value of the crossing as preserving continuity in the map of the visual world. O is the optic nerve and C is the superior colliculus or optic tectum (the names are synonymous), (c) This is Ariens-Kapper's picture of the cross section of the brain of a frog through the colliculus, which is the upper or dorsal part above the enclosed space, (d) This is Pedro Ramon Cajal's diagram of the nervous organization of the tectum of a frog. The terminal bushes of the optic nerve fibers are labelled a, b, and c. A, B, C, D and E are tectal cells receiving from the optic nerve fibers. Note that the axons of these cells come off the dendrites in stratum 7, which we call the palisade layer. The endings discussed in this paper lie between the surface and that stratum.
If the small spot of light suddenly appears or disappears within the field of an ON-OFF cell, the discharge is short and occurs in both cases.
If the spot of light disappears from the field of an OFF cell, the discharge begins immediately, decreases slowly in frequency, and lasts a long time. It can be abolished promptly by turning the spot of light on again.
For all three sorts of field, sensitivity is greatest at the center of each field and least at the periphery.
Barlow(5) extended Hartline's observations. He observed that the OFF cells have an adding receptive field, the response occurs always to OFF at both center and periphery of that field, and that the effect of removing light from the periphery adds to the effect of a reduction of light at the center, with a weight decreasing with distance.
The ON-OFF cells, however, have differencing receptive fields. A discharge of several spikes to the appearance of light in the center is much diminished if a light is turned on in the extreme periphery. The same interaction occurs when these lights are removed. Thus, an ON-OFF cell seems to be measuring inequality of illumination within its receptive field. (Kuffler(4) at the same time showed a similar mutual antagonism between center and periphery in each receptive field of ganglion cells in the eye of a cat, and later Barlow, Kuffler and Fitzhugh(6) showed that the size of the cat's receptive fields varied with general illumination.) Barlow saw that ON-OFF cells were profoundly sensitive to movement within the receptive field. The ON cells have not been characterized by similar methods.
These findings of Hartline and Barlow establish that optic nerve fibers (the axons of the ganglion cells) do not transmit information only about light intensity at single points in the retina. Rather, each fiber measures a certain feature of the whole distribution of light in an area of the receptive field. There are three sorts of function, or areal operation, according to these authors, so that the optic nerve looks at the image on the retina through three distributed channels. In any one channel, the overlap of individual receptive fields is very great. Thus one is led to the notion that what comes to the brain of a frog is this: for any visual event, the OFF channel tells how much dimming of light has occurred and where; the ON-OFF channel tells where the boundaries of lighted areas are moving, or where local inequalities of illumination are forming; the ON channel shows (with a delay) where brightening has occurred. To an unchanging visual pattern, the nerve ought to become fairly silent after a while.
Consider the retinal image as it appears in each of the three distributed channels. For both the OFF and ON channels, we can treat the operation on the image by supposing that every point on the retina gives rise to a blur about the size of a receptive field. Then the OFF channel tells, with a long decay time, where the blurred image is darkened, and the ON channel tells with a delay and long decay where it is brightened. The third channel, ON-OFF. principally registers moving edges. Having the mental picture of an image as it appears through the three kinds of channel, we are still faced with the question of how the animal abstracts what is useful to him from his surroundings. At this point, a safe position would be that a fair amount of data reduction has in fact been accomplished by the retina and that the interpretation is the work of the brain, a yet- to-be unraveled mystery. Yet the nagging worries remain: why are there two complementary projections of equally poor resolution? Why is the mosaic of receptors so uselessly fine?
Initial Argument
The assumption has always been that the eye mainly senses light, whose local distribution is transmitted to the brain in a kind of copy by a mosaic of impulses. Suppose we held otherwise, that the nervous apparatus in the eye is itself devoted to detecting certain patterns of light and their changes, corresponding to particular relations in the visible world. If this should be the case, the laws found by using small spots of light on the retina may be true and yet, in a sense, be misleading. Consider, for example, a bright spot appearing in a receptive field. Its actual and sensible properties include not only intensity, but the shape of its edge, its size, curvature, contrast, etc.
We decided then how we ought to work. First, we should find a way of recording from single myelinated and unmyelinated fibers in the intact optic nerve. Second. we should present the frog with as wide a range of visible stimuli as we could, not only spots of light but things he would be disposed to eat. other things from which he would flee, sundry geometrical figures, stationary and moving about, etc. From the variety of stimuli we should then try to discover what common features were abstracted by whatever groups of fibers we could find in the optic nerve. Third, we should seek the anatomical basis for the grouping.3
(Actual) Methods
Using a variant of Dowben and Rose's platinum black-tipped electrode described in another paper of this issue, we then began a systematic study of fibers in the optic nerve. One of the authors (H R M.) had completed the electron microscopy of optic nerve in frogs,(7) and with his findings we were able to understand quickly why certain kinds of record occurred. He had found that the optic nerve of a frog contains about half a million fibers (ten times the earlier estimates by light microscopy) There are 30 times as many unmyelinated axons as myelinated, and both kinds are uniformly distributed throughout the nerve. The axons lie in small densely packed bundles of five to 100 fibers with about 100 A between axons, each bundle surrounded by one or more glial cells.(7) But along the nerve no bundle maintains its identity long, for the component fibers so braid between bundles that no two fibers stay adjacent. Thus the place a fiber crosses one section of the nerve bears little relation to its origin in the retina and little relation to where it crosses another section some distance away.
Fibers are so densely packed that one might suppose such braiding necessary to prevent serious interactions. On the other hand, the density makes the recording easier. A glial wall surrounds groups rather than single fibers, and penetration of the wall brings the tip among really bare axons each surrounded by neighbors whose effect is to increase the external impedance to its action currents, augmenting the external potential in proportion. Thus, while we prefer to use platinum black tips to improve the ratio of signal to noise, we recorded much the same population with ordinary sharp microelectrodes of bright Pt or Ag. The method records equally well from unmyelinated and myelinated fibers.
We used Ranapipiens in these experiments. We opened a small flap of bone either just behind the eye to expose the optic nerve, or over the brain to expose the superior colliculus. No further surgery was done except to open the membranes of connective tissue overlying the nervous structure. The frog was held in extension to a cork platform and covered with moist cloth. An animal in such a position, having most of his body surface in physical contact with something, goes into a still reaction—i.e., he will not even attempt to move save to pain, and except for the quick small incision of the skin at the start of the operation our procedure seems to be painless to him. With the animal mounted, we confront his eye with an aluminum hemisphere, 20 mils thick and 14 inches in diameter, silvered to a matte grey finish on the inner surface and held concentric to the eye. On the inner surface of this hemisphere, various objects attached to small magnets can be moved about by a large magnet moved by hand on the outer surface. On our hemisphere, 1° is slightly less than an eighth of an inch long. In the tests illustrated, we use as stimulating objects a dull black disk almost 1® in diameter and a rectangle 30° long and 12° wide. However, in the textual report, we use a variety of other objects. As an indicator for the stimulus, we first used a phototube looking at an image of the hemisphere taken through a camera lens and focused on the plane of a diaphragm. (Later we used a photomultiplier, so connected as to give us a logarithmic response over about 4 decades.) Thus we could vary how much of the hemisphere was seen by the stimulus detector and match that area in position and size against the receptive field of the fiber we were studying. The output of this arrangement is the stimulus line in the figures.
Findings
There are four separate operations on the image in the frog's eye. Each has its result transmitted by a particular group of fibers, uniformly distributed across the retina, and they are all nearly independent of the general illumination. The operations are: 1) sustained contrast detection; 2) net convexity detection;3) moving edge detection; and 4) net dimming detection. The first two are reported by unmyelinated fibers, the last two by myelinated fibers. Since we are now dealing with events rather than point excitations as stimuli, receptive fields can only be defined approximately, and one cannot necessarily distinguish concentric subdivisions. The fibers reporting the different operations differ systematically not only in fiber diameter (or conduction velocity) but also in rough size of receptive field, which ranges from about 2° diameter for the first operation, to about 15° for the last. The following description of these groups is definite.
Sustained Contrast Detectors
In Fig. 2 we see the response of such a fiber in the optic nerve. We compare these responses with full illumination (a 60-watt bulb and reflector mounted a foot away from the plane of the opening of the hemisphere) to those with less than 1/300 as much light (we put a variable resistance in series with the bulb so that the color changed also). We are struck by the smallness of the resulting change. In very dim light where we can barely see the stimulating object ourselves, we still get very much the same response.
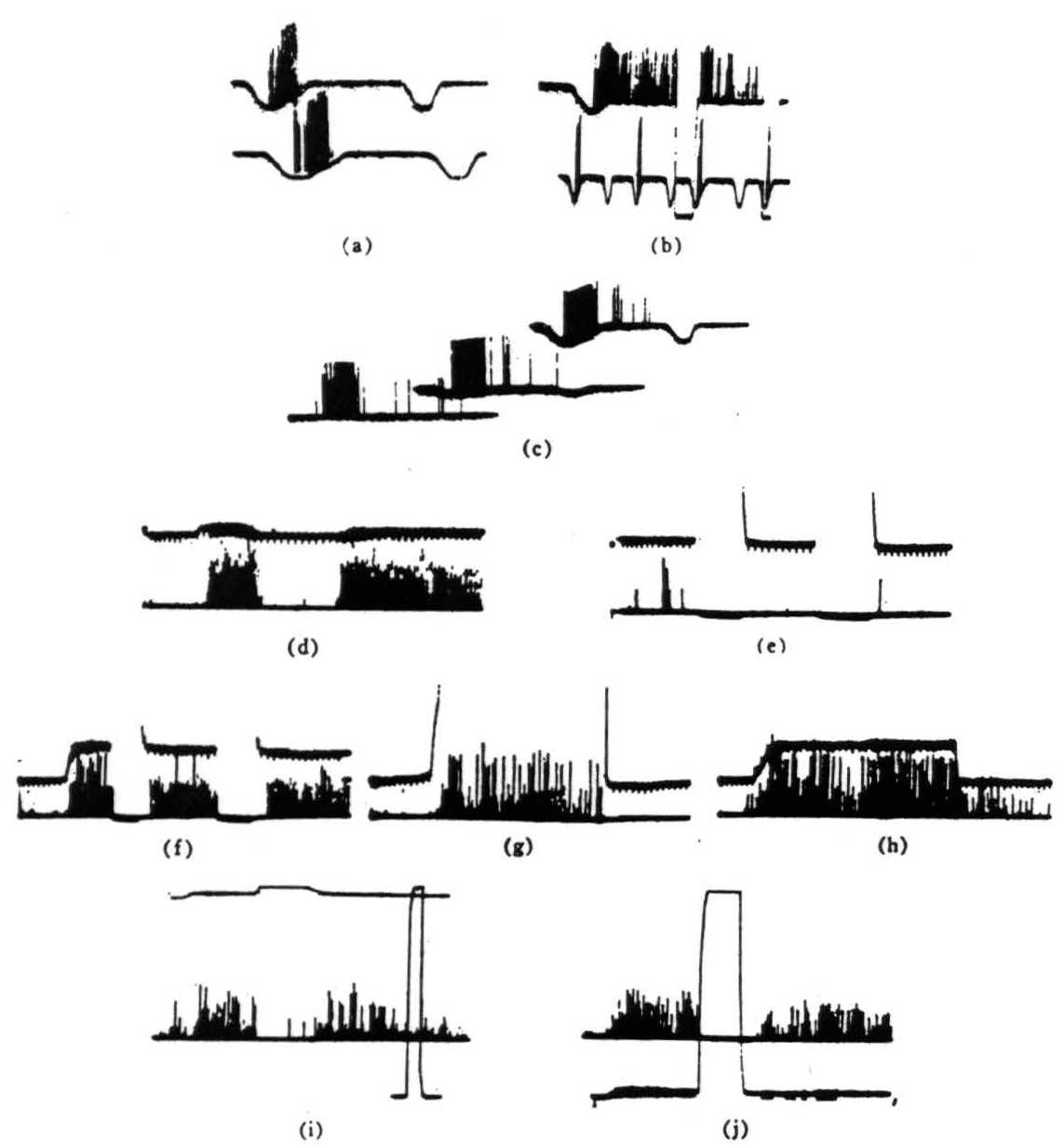
Figure 2. Operation 1)—contrast detectors. The records were all taken directly with a Polaroid camera. The spikes are clipped at the lower end just above the noise and brightened on the screen. Occasional spikes have been intensified by hand for purposes of reproduction. The resolution is not good but we think that the responses are not ambiguous. Our alternate recording method is by means of a device which displays the logarithm of pulse interval of signals through a pulse height pick-off. However, such records would take too much explanation and would not add much to the substance of the present paper, (a) This record is from a single fiber in the optic nerve. The base line is the output of a photocell watching a somewhat larger area than the receptive field of the fiber. Darkening is given by downward deflection. The response is seen with the noise clipped off. The fiber discharge to movement of the edge of a 3° black disk passed in one direction but not to the reverse movement. (Time marks, 20 per second.) (b) The same fiber shown here giving a continued response when the edge stops in the field. The response disappears if the illumination is turned off and reappears when it is turned on. Below is shown again the asymmetry of the response to a faster movement. (Time marks. 20 per second.) (c) The same fiber is stimulated here to show asymmetrical response to the 3° black object moved in one direction, then the reverse and the stimuli are repeated under a little less than a 3-decade change of illumination in two steps. The bottom record is in extremely dim light, the top in very bright light. (Time marks, 20 per second.) (d) In the bottom line, a group of endings from such fibers is shown recorded from the first layer in the tectum A black disk 1° in diameter is moved first through the field and then into the field and stopped. In the top line, the receptive field is watched by a photomultiplier (see text) and darkening is given by upward deflection. (Time marks, .5 per second for all tectal records.) (e) OFF and ON of general illumination has no effect on these fibers, (f) A 3° black disk is moved into the field and stopped. The response continues until the lights are turned OFF but reappears when the lights are turned ON. These fibers are nonerasable, (g) A very large black square is moved into the field and stopped. The response to the edge continues so long as the edge is in the field, (h) The 3° disk is again moved into the field and stopped. When it leaves, there is a slight after-discharge, (i) A 1° object is moved into the field, stopped, the light is then turned off, then on. and the response comes back. The light is, however, a little less than 300X dimmer than in the next frame. Full ON and OFF are given in the rectangular calibration on the right, (j) The same procedure as in Fig. 2(i) is done under very bright light. The return of response after reintroducing the light seems more prolonged—but this is due only to the fact that, in Fig. 2(i), the edge was not stopped in optimal position.
Net Convexity Detectors
These fibers form the other subdivision of the unmyelinated population, and require a number of conditions to specify when they will respond. To our minds, this group contains the most remarkable elements in the optic nerve.
Such a fiber does not respond to change in general illumination. It does respond to a small object (3° or less) passed through the field; the response does not outlast the passage. It continues responding for a long time if the object is imported and left in the field, but the discharge is permanently turned off (erased) by a transient general darkness lasting 1/10 second or longer. We have not tried shorter obscurations.
The fiber will not respond to the straight edge of a dark object moving through its receptive field or brought there and stopped. If the field is about 7° in diameter, then, if we move a dark square 8° on the side through it with the edge in advance there is no response, but if the comer is in advance then there is a good one. Usually a fiber will respond indefinitely only to objects which have moved into the field and then lie wholly or almost wholly interior to the receptive field. The discharge is greater the greater the convexity, or positive curvature, of the boundary of the dark object until the object becomes as small as about ½ the width of the receptive field. At this point, we get the largest response on moving across that field, and a good, sustained response on entering it and stopping. As one uses smaller and smaller objects, the response to moving across the field begins to diminish at a size of about 1°, although the sustained response to coming in and stopping remains. In this way we find the smallest object to which these fibers respond is less than 3 minutes of arc. A smooth motion across the receptive field has less effect than a jerky one, if the jerks recur at intervals longer than ½ second. A displacement barely visible to the experimenter produces a marked increase in response which dies down slowly.
Any checked or dotted pattern (in the latter case, with dots no further apart than half the width of the receptive field) moved as a whole across the receptive field produces little if any response. However, if any dot within the receptive field moves differentially with respect to the background pattern, the response is to that dot as if it were moving alone. A group of two or three distinct spots enclosed within the receptive field and moved as a whole produce less direct response to movement and much less sustained response on stopping than if the spots are coalesced to a single larger spot.
A delightful exhibit uses a large color photograph of the natural habitat of a frog from a frog's eye view, flowers and grass. We can move this photograph through the receptive field of such a fiber, waving it around at a 7-inch distance: there is no response. If we perch with a magnet a fly-sized object 1° large on the part of the picture seen by the receptive field and move only the object we get an excellent response. If the object is fixed to the picture in about the same place and the whole moved about, then there is none.
Finally, the response does not depend on how much darker the object is than its background, so long as it is distinguishably so and has a clear-cut edge. If a disk has a very dark center and merges gradually into the grey of the background at the boundary, the response to it is very much less than to a uniform grey disk only slightly darker than the background. Objects lighter than the background produce almost no response unless they have enough relief to cast a slight shadow at the edge.
All the responses we have mentioned are relatively independent of illumination, and Fig. 3 taken as described in the caption shows the reactions to a 3° object and the large rectangle under some of the conditions described.
General Comments on Groups 1) and 2)
The two sorts of detectors mentioned seem to include all the unmyelinated fibers, with conduction velocities of 20 to 50 cm. The two groups are not entirely distinct. There are transition cases. On one hand, some convexity detectors respond well to very slightly curved edges, even so far as to show an occasional sustained response if that edge is left in the field. They may also not be completely erasable (though very markedly affected by an interruption of light) for small objects. On the other hand, others of the same group will be difficult to set into an indefinitely sustained response with any object, but only show a fairly long discharge, acting thereby more as detectors of edges although never reacting to straight edges. Nevertheless the distribution of the unmyelinated axons into two groups is very marked. Any fiber of either group may show a directional response—i.e., there will be a direction of movement that may fail to excite the cell. For the contrast fibers, this will also appear as a nonexciting angle of the boundary with respect to the axis of the frog. Such null directions and angles cancel out in the aggregate.
Moving-Edge Detectors
These fibers are myelinated and conduct at a velocity in the neighborhood of 2 meters per second. They are the same as Hartline's and Barlow's ON-OFF units. The receptive field is about 12° wide. Such a fiber responds to any distinguishable edge moving through its receptive field, whether black against white or the other way around. Changing the extent of the edge makes little difference over a wide range, changing its speed a great one. It responds to an edge only if that edge moves, not otherwise. If an object wider than about 5° moves smoothly across the field, there are separate responses to the leading and trailing edges, as would be expected from Barlow's formulation. These fibers generally show two or three regularly spaced spikes, always synchronous among different fibers to turning the light on or off or both. The response to moving objects is much greater than to changes in total illumination and varies only slightly with general illumination over a range of 1/300. The frequency of the discharge increases with the velocity of the object within certain limits (see Fig. 4).
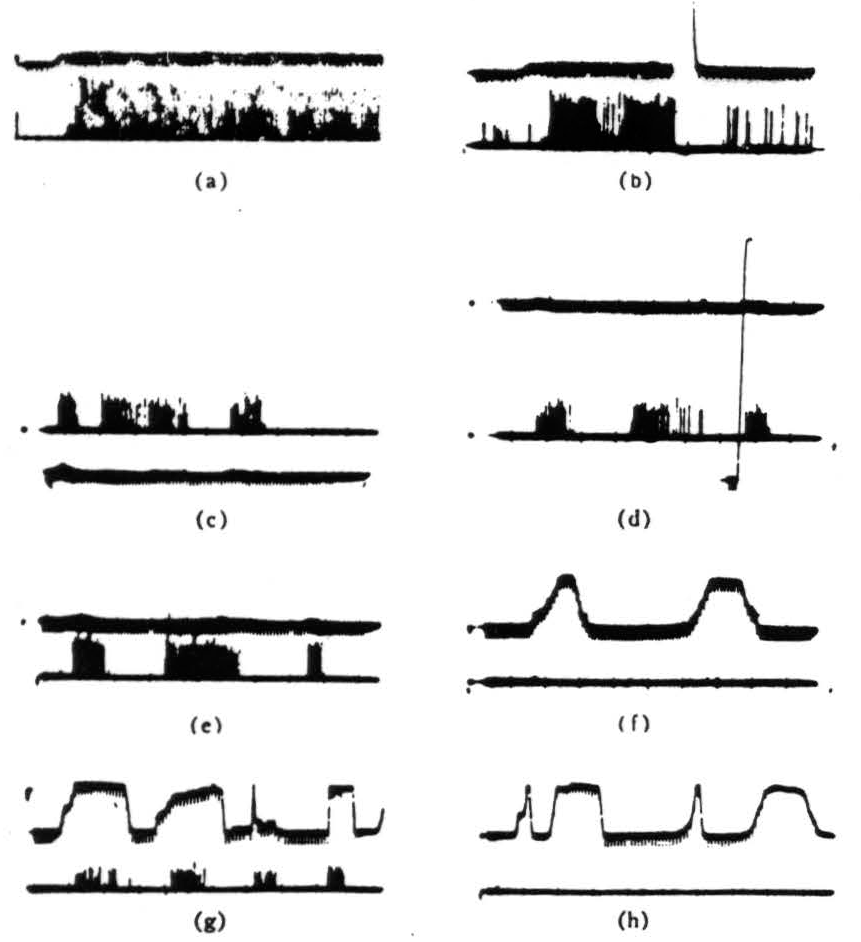
Figure 3. Operation 2)—convexity detectors. The photomultiplier is used, and darkening is an upward deflection, (a) These records are all from the second layer of endings in the tectum. In the first picture, a 1° black disk is imported into the receptive field and left there. (b)The same event occurs as in Fig. 3(a), but now the light is turned off then on again. The response is much diminished and in the longer record vanishes. These fibers are erasable. (c) The 1° disk, is passed through the held first somewhat rapidly, then slowly, then rapidly. The light is very bright, (d) The same procedure occurs as in Fig. 3(c), but now the light has been dimmed about 300×. The vertical line shows the range of the photomultiplier which has been adjusted for about 3½ decades of logarithmic response, (e) A 1° black disk is passed through the held at three speeds, (f) A 15° black strip is passed through at two speeds edge leading, (g) A 15° black strip is passed through in various ways with corner leading, (h) The same strip as in Fig. 3(g) is passed through, edge leading.
Net Dimming Detectors
These are Hartline's and Barlow's OFF fibers. But they have some properties not observed before. They are myelinated and the fastest conducting afferents, clocked at 10 meters per second.4 One such fiber responds to sudden reduction of illumination by a prolonged and regular discharge. Indeed, the rhythm is so much the same from fiber to fiber that in recording from several at once after sudden darkening, the impulses assemble in groups, which break up only after many seconds. Even then the activity from widely separated retinal areas seems to be related. We observe that the surface potential of the colliculus shows a violent and prolonged oscillation when the light is turned off. This oscillation, beginning at about 18 per second and breaking into 3 to 5 per second after several seconds, seems to arise from these fibers from the retina; the same record is seen when the optic nerve is severed and the recording electrode placed on the retinal stump. See Fig. 5.
The receptive field is rather large—about 15°—and works as Barlow describes. Darkening of a spot produces less response when it is in the periphery of the field than when it is at the center. The effect of a moving object is directly related to its size and relative darkness. The response is prolonged if a large dark object stops within the held. It is almost independent of illumination, actually increasing as the light gets dimmer. There is a kind of erasure that is complementary to that of group 2). If the general lighting is sharply dimmed, but not turned off entirely, the consequent prolonged response is diminished or abolished after a dark object passes through the receptive held. In this case, the reasons for erasure are apparent. Suppose one turns off the light and sets up a prolonged response. Then the amount of light which must be restored to interrupt the response gets less and less the longer one waits. That is, the sensitivity of the OFF discharge to the ON of light increases with time. If we darken the general lighting only by a factor of 100, we also get a prolonged discharge. However, if we turn off the light completely a few seconds after the 100/1 dimming and then turn it back on to the same dim level, the discharge is increased by the second dimming and is completely or almost completely abolished by the relighting. The effect of moving a dark object through the field after dimming is to impose a second dimming pulse followed by brightening as the object passes.
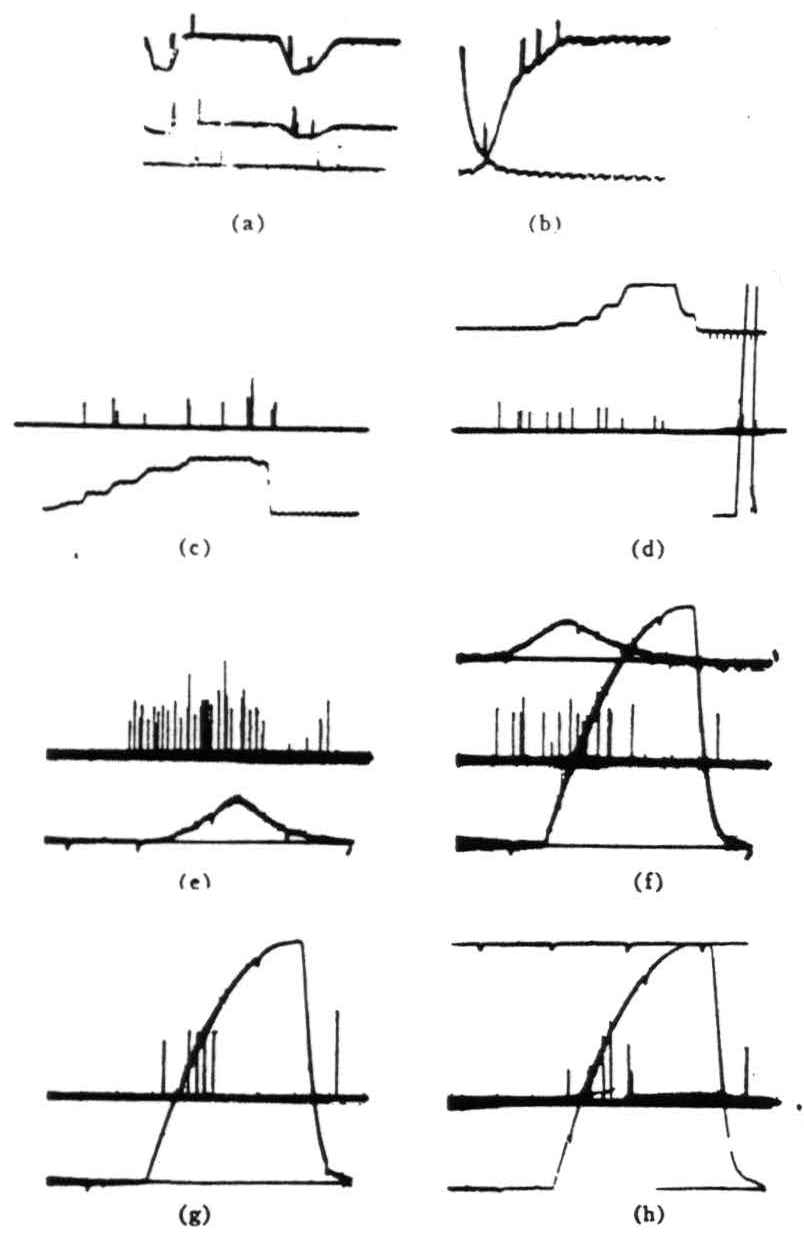
Figure 4. Operation 3)—moving-edge detector. The first two pictures are taken from a single fiber in the optic nerve, (a) Shows a 7° black disk moving through the receptive field (the photocell was not in registration with the field). There is a response to the front and back of the disk independent of illumination. There is about a 300/1 shift of illumination between top and bottom of the record. Darkening is a downward deflection with the photocell record. (Time marks, 5 per second.) (b) OFF and ON of general lighting. (Time marks, 50 per second.) Note double responses and spacing, (c) This and succeeding records are in the third layer of endings in the tectum. Several endings are recorded but not resolved. Darkening is an upward deflection of the photomultiplier record. The response is shown to the edge of a 15° square moved into and out of the field by jerks in bright light, (d) The same procedure occurs as in Fig. 4(c), but in dim light. Calibration figure is at the right, (e) The response is shown to a 7° black disk passed through the receptive fields under bright light. The sweep is faster, but the time marks are the same, (f) The same procedure as for Fig. 4(e), but under dim light. The OFF and ON of the photomultiplier record was superimposed for calibration, (g) OFF and ON response with about half a second between ON and OFF. (h) Same as Fig. 4(g), but with 2 seconds between OFF and ON.
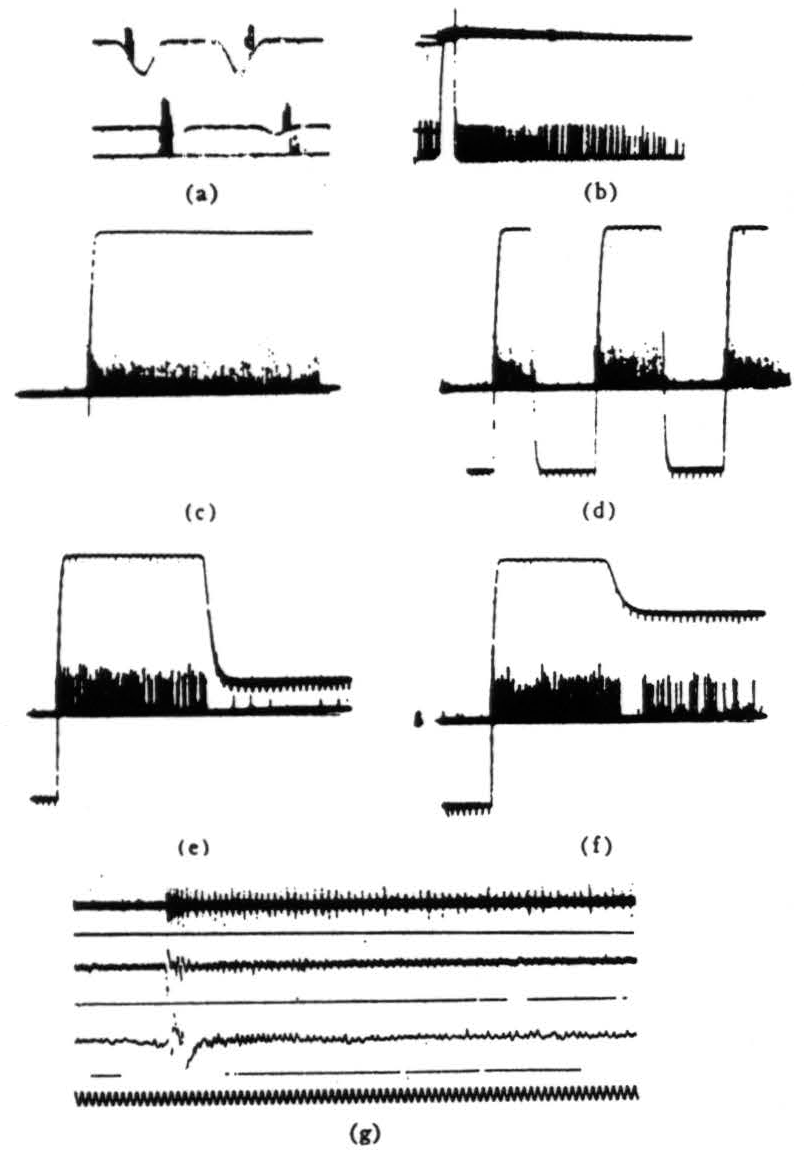
Figure 5. Operation 4)—Dimming detectors, (a) This and the next frame are taken from a single fiber in the optic nerve. Here we see the response to a 7° black disc passing through the receptive field. The three records are taken at three illumination levels over a 300:1 range. In the phototube record, darkening is a downward deflection. (Time marks, 5 per second.) (b) OFF and ON of light. The OFF was done shortly after one sweep began, the ON occurred a little earlier on the next sweep. The fiber is silenced completely by the ON. (Time marks 5 second.) (c) In this and the next three frames, we are recording from the fourth layer of endings in the tectum. This frame shows the response to turning OFFs the general illumination, (d) OFF and ON of light at regular intervals, (e) OFF then on of the light to a lesser brightness. (f) OFF the ON of the light to a still lesser brightness, (g) The synchrony of the dimming detectors as described in the text. At the top are three or four fibers recorded together in the optic nerve when the light is suddenly turned off. The fibers come from diverse areas on the retina. In the second line are the oscillations recorded from the freshly cut retinal stump of the optic nerve when the light is suddenly turned off. In the third line are the oscillations recorded on the surface of the tectum, the visual brain, before the nerve was cut. Again the light is suddenly turned off. The last line is 20 cps. These records of synchrony were obviously not all made at the same time, so that comparing them in detail is not profitable.
Others
Lastly, there is a small group of afferent fibers which does not seem to have distinct receptive fields. They each measure the absolute degree of darkness over a wide area with a long time constant. That is, the frequency of discharge is greater the darker it is. They have a complement in that some of the moving edge detectors have a resting discharge of very low frequency if the illumination is extremely bright
Discussion
Let us compress all of these findings in the following description. Consider that we have four fibers, one from each group, which are concentric in their receptive fields.
Suppose that an object is moved about in this concentric array:
The contrast detector tells, in the smallest area of all, of the presence of a sharp boundary, moving or still, with much or little contrast.
The convexity detector informs us in a somewhat larger area whether or not the object has a curved boundary, if it is darker than the background and moving on it; it remembers the object when it has stopped, providing the boundary lies totally within that area and is sharp; it shows most activity if the enclosed object moves intermittently with respect to a background. The memory of the object is abolished if a shadow obscures the object for a moment.
The moving-edge detector tells whether or not there is a moving boundary in a yet larger area within the field.
The dimming detector tells us how much dimming occurs in the largest area, weighted by distance from the center and by how fast it happens.
All of the operations are independent of general illumination. There are 30 times as many of the first two detectors as of the last two, and the sensitivity to sharp-ness of edge or increments of movement in the first two is also higher than in the last two.
Results in the Tectum
As remarked earlier, the optic nerve fibers are all dis-ordered in position through the nerve. That is, the probability that any two adjacent fibers look at the same region in the retina is very small. However, when the fibers terminate in the superior colliculus they do so in an orderly way such that the termini exhibit a continuous map of the retina. Each optic nerve crosses the base of the skull and enters the opposite tectum [Fig. 1(b)] via two bundles—one rostromedial, the other caudalateral. The fibers sweep out over the tectum in the superficial neuropil in what grossly appears to be a laminated way [Fig. 1(c) ]. The detail of ending is not known, and there is some reason to think Pedro Ramon's drawing(3) is too diagrammatic [Fig. 1(d) ], however well it fits with our data.
In any case, the outer husk of neuropil, roughly about half the thickness of the optic tectum, is formed of the endings of the optic fibers mixed with dendrites of the deeper lying cells, and in this felting lie few cell bodies.
We have found it singularly easy to record from these terminal bushes of the optic fibers. That is, if an electrode is introduced in the middle of one bush, the external potential produced by action currents in any branch increases in proportion to the number of branches near the electrode. Since the bushes are densely interdigitated everywhere, it is not difficult to record from terminal arbors anywhere unless one kills or blocks them locally, as is easily done by pressure, etc.
One may inquire how we can be sure of recording from terminal arbors, and not from cells and their dendrites. The argument is this. First, there are about four layers of cells in the depths of the tectum [Fig. 1(d) ], and only their dendrites issue into the superficial neuropil wherein lie very few cells indeed. There arc about 250,000 of these cells in all, compared to 500,000 optic fibers. In the outer thickness of the tectum, among the terminating fibers, almost every element we record performs one of the four operations characterizing the fibers in the nerve, and has a corresponding receptive field. Now as the electrode moves down from the surface in one track, we record 5 to 10 cells in the deepest half of the tectum. Not a single cell so recorded shows activity even remotely resembling what we find in the superficial neuropil. Among the cells, none show optic nerve operations, and the smallest receptive fields we find are over 30° in diameter. If the active elements in the upper layers are cells (one will see about 20 to 30 active elements in one electrode track before reaching the cell layer), which cells can they be? Or if they are dendrites, to what neurons do they belong? We regard these considerations as conclusive enough.
Figs. 2-5 show that the four operational groups of fibers terminate in four separate layers of terminals, each layer exhibiting a continuous map of the retina (we confirm Gaze's diagram of the projection(8)) and all four maps are in registration. Most superficial lie the endings for the contrast detectors, the slowest fibers. Beneath them, but not so distinctly separate, are the convexity detectors. Deeper, and rather well separated, are the moving-edge detectors admixed with the rare and ill-defined axons that measure the actual level of darkness. Deepest (and occasionally contaminated with tectal cells or their axons) lie the dimming detectors. Thus the depth at which these fibers end is directly related to their speed of conduction.
Such an arrangement makes experiment easy, for all the fibers of one operation performed on the same field in the retina end in one place in the tectum and can be recorded as a group. It is very useful to see them this way, for then the individual variations among similar units cancel one another and only the common properties remain. We made the tectal records shown in the accompanying figures with a single electrode in two penetrations (to get decent separation of contrast and convexity detectors which lie just below the pia), to show how clear-cut the arrangement is.
Confirmation of Sperry's Proposal
The existence of a fourfold map of the retina in the tectal neuropil led us, naturally, to repeat Sperry's initial experiment on the regeneration of cut optic nerve.(9) Since the nerve is as scrambled as it can be originally, we saw no point in turning the eye around 180° but simply cut one nerve in a few frogs, separated the stumps to be sure of complete severance, and waited for about 3 months. At the end of this time, after it was clear that the cut nerves were functioning again, we compared the tectal maps of the cut and uncut nerves in some of them. We confirmed (as did Gaze(10)) Sperry's proposal that the fibers grew back to the regions where they originally terminated in mapping the retina.(11) But we also found a restoration of the four layers with no error or mixing. In one frog, after 90 days, the fibers had grown back best at the entrance of the two brachia to the colliculus, and least at the center, yet there were no serious errors. The total area of retina communicating with one point of the collicular neuropil (i.e, the sum of the receptive fields of the fibers recorded from that point) had increased three or four times, from a diameter of about 15° to a diameter of about 30°. But there was no admixture of fibers with receptive fields in widely separated regions. In another frog, after 120 days, the area seen from one point was barely twice normal.
General Discussion
What are the consequences of this work? Fundamentally, it shows that the eye speaks to the brain in a language already highly organized and interpreted, instead of transmitting some more or less accurate copy of the distribution of light on the receptors. As a crude analogy, suppose that we have a man watching the clouds and reporting them to a weather station if he is using a code, and one can see his portion of the sky too. then it is not difficult to find out what he is saying. It is certainly true that he is watching a distribution of light; nevertheless, local variations of light are not the terms in which he speaks nor the terms in which he is best understood. Indeed, if his vocabulary is restricted to types of things that he sees in the sky, trying to find his language by using flashes of light as stimuli will certainly fail. Now, since the purpose of a frog's vision is to get him food and allow him to evade predators no matter how bright or dim it is about him, it is not enough to know the reaction of his visual system to points of light. To get useful records from individual receptors (the rods and cones), assuming that they operate independently and under no reflex control, this stimulus may be adequate. But when one inspects responses that are a few nervous transformations removed from the receptors, as in the optic nerve, that same choice of stimulus is difficult to defend. It is equivalent to assuming that all of the interpretation is done further on in the nervous system. But, as we have seen, this is false.
One might attempt to measure numerically how the response of each kind of fiber varies with various properties of the successions of patterns of light which evoke them. But to characterize a succession of patterns in space requires knowledge of so many independent variables that this is hardly possible by experimental enumeration of cases. To examine the effect of curvature alone we should have to explore at least the response to all configurations of three spots moving in a large variety of ways. We would prefer to state the operations of ganglion cells as simply as possible in terms of whatever quality they seem to detect and, instead, examine the bipolar cells in the retina, expecting to find there a dissection of the operations into combinations of simpler ones performed at intermediate stages. For example, suppose that there are at least two sorts of rods and cones, one increasing its voltage with the log of light at one color, the other decreasing its voltage with the log of light at some other color. If bipolars connect to several contiguous rods or cones of opposing reactions and simply add voltages, some bipolars will register a large signal only if an appropriate contrast occurs. We have in fact found something of the sort occurring, for it seems that the inner plexiform layer of the retina is stratified to display several different local properties, one layer indicating local differences in intensity of light. Some of Svaetichin's(12) data can be adduced here. The different dendritic distribution of the ganglion cells, as in Fig. 1(a), may signify that they extract differently weighted samples of simple local operations done by the bipolars, and it is on this that we are now working.
But there is another reason for a reluctance to make accurate measurements on the activity of ganglion cells in the intact animal. A significant efferent outflow goes to the retina from the brain. We now know to a certain extent how the cells in the tectum handle the four inputs to them which are described in this paper. There are at least two distinct classes of these cells, and at least one of them issues axons back into the optic nerve. We have recorded this activity there. Such axons enter the retina and we think some effects of their activity on the sensitivity of ganglion cells are noticeable.
The way that the retina projects to the tectum suggests a nineteenth century view of visual space. The image on the retina, taken at the grain of the rods and cones, is an array of regularly spaced points at each of which there is a certain amount of light of a certain composition. If we know the position of every point and the values of light at every point, we can physically reconstruct the image, and looking at it understand the picture. If, however, we are required to establish continuities within the picture only from the numerical data on position and light at independent points, it becomes a very difficult task. The retina projects onto the tectum in four parallel sheets of endings, each sheet mapping continuously the retina in terms of a particular areal operation, and all four maps are in registration. Consider the dendrite of a tectal cell extending up through the four sheets. It is looking at a point in the image on the retina, but that point is now seen in terms of the properties of its neighborhood as defined by the operations. Since the overlap of receptive fields within any operation is very great, it now seems reasonable to erect simple criteria for finding continuities. For example, if an area over which there is little change in the fourfold signature of a moving object is bounded by regions of different signature, it seems likely that that area describes the image of a single object.
By transforming the image from a space of simple discrete points to a congruent space where each equivalent point is described by the intersection of particular qualities in its neighborhood, we can then give the image in terms of distributions of combinations of those qualities. In short, every point is seen in definite contexts. The character of these contexts, genetically built in, is the physiological synthetic a priori. The operations found in the frog make unlikely later processes in his system of the sort described by two of us earlier(13). for example, dilatations; but those were adduced for the sort of form recognition which the frog does not have. This work is an outgrowth of that earlier study which set the question.
Conclusion
The output from the retina of the frog is set of four distributed operations on the visual image. These operations are independent of the level of general illumination and express the image in terms of: 1) local sharp edges and contrast: 2) the curvature of edge of a dark object; 3) the movement of edges; and 4) the local dimmings produced by movement or rapid general darkening. Each group of fibers serving one operation maps the retina continuously in a single sheet of endings in the frog's brain. There are four such sheets in the brain, corresponding to the four operations, and their maps are in registration. When all axonal connections between eye and brain are broken and the fibers grow back, they reconstitute the original retinal maps and also arrange themselves in depth in the original order with no mistakes. If there is any randomness in the connections of this system it must be at a very fine level indeed. In this, we consider that Sperry(9) is completely right.
We have described each of the operations on the retinal image in terms of what common factors in a large variety of stimuli cause response and what com-mon factors have no effect. What, then, does a particular fiber in the optic nerve measure? We have considered it to be how much there is in a stimulus of that quality which excites the fiber maximally, naming that quality.
The operations thus have much more the flavor of perception than of sensation if that distinction has any meaning now. That is to say that the language in which they are best described is the language of complex abstractions from the visual image. We have been tempted, for example, to call the convexity detectors “bug perceivers.” Such a fiber [operation 2] responds best when a dark object, smaller than a receptive field, enters that field, stops, and moves about intermittently thereafter. The response is not affected if the lighting changes or if the background (say a picture of grass and flowers) is moving, and is not there if only the background, moving or still, is in the field. Could one better describe a system for detecting an accessible bug?
Acknowledgment
We are particularly grateful to O. G. Selfridge whose experiments with mechanical recognizers of pattern helped drive us to this work and whose criticism in part shaped its course.
Bibliography
H. R. Maturana, “Number of fibers in the optic nerve and the number of ganglion cells in the retina of Anurans,” Nature, vol. 183, pp 1406-1407; May 16. 1959.
H. K. Hartline, “The response of single optic nerve fibres of the vertebrate eye to illumination of the retina,” Amer. J. Physiol., vol. 121, pp. 400-415; February, 1938. Also, “The receptive fields of the optic nerve fibers,” Amer. J. Physiol., vol. 130, pp. 690-699; October, 1940.
Pedro Ramon Cajal, “Histologie du Systeme Nerveux,” Ramon y Cajal, Maloine. Paris, France; 1909-1911.
S. W. Kuffler, “Discharge patterns and functional organization of mammalian retina,” J. Neurophysiol., vol. 16. pp. 37-68; January, 1953.
H. B. Barlow, “Summation and inhibition in the frog's retina,” J. Physiol., vol. 119, pp. 69-88; January, 1953.
H. B. Barlow, R. Fitzhugh, and S. W. Kuffler, “Change of organization in the receptive fields of the cat's retina during dark adaptation,” J. Physiol., vol. 137, pp. 338-354; August, 1957.
H. R. Maturana, “The Fine Structure of the Optic Nerve and Tectum of Anurans. An Electron Microscope Study,” Ph.D. dissertation, Harvard University, Cambridge, Mass.; 1958.
R. M. Gaze, “The representation of the retina on the optic lobe of the frog,” Quart. J. Exper. Physiol., vol. 43, pp. 209-214; March. 1958.
R. Sperry, “Mechanisms of neural maturation,” in “Handbook of Experimental Psychology,” S. S. Stevens, Ed., John Wiley and Sons, Inc., New York. N. Y.; 1951.
R. M. Gaze, “Regeneration of the optic nerve in Xenopus laevi,” J. Physiol., vol. 146, p. 40P; February 20-21, 1959.
H. R. Maturana, J. Y. Lettvin, W. S. McCulloch, and W. H. Pitts, “Physiological evidence that cut optic nerve fibers in the frog regenerate to their proper places in the tectum,” Science: 1959 (in press).
G. Svaetichin and E. F. NcNichol Jr., “Retinal mechanisms for chromatic and achromatic vision.” Ann. N. Y. Acad. Sci., vol. 74, pp. 385-404: November, 1958.
W. S. McCulloch and W. H. Pitta, “How we know universals. The perception of auditory and visual forms,” Bull. Math. Biophysics, vol. 9. pp. 127-147; June. 1947.
A. M. Andrew, “Report on Frog Colliculus,” Res. Lab. of Electronics, Mass. Inst. Tech., Cambridge. Quarterly Progress Rept.. pp. 77-78; July 15. 1955.
A. M. Andrew, “Action potentials from the frog colliculus,” J. Physiol., vol. 130, p. 25P; September 23-24, 1955.