FACTORS FOR FACILITATION AND EXTINCTION IN THE CENTRAL NERVOUS SYSTEM12 [26]
J.G. Dusser de Barenne and W.S. McCulloch
Introduction
The Phenomenon now known as “facilitation” was discovered in 1881 by Bubnoff and Heidenhain(1) who observed that, with an interval of only a few seconds, repetition of supraliminal stimulation of a “motor” focus of the cerebral cortex elicited a larger peripheral response with shorter latency, and even that repetition of subliminal stimulation could bring in a response. They also demonstrated augmentation of motor response to cortical stimulation by antecedent cutaneous stimulation of the limb in which the response occurred. These phenomena are called facilitation. Facilitation of motor response can also be induced by antecedent stimulation of a neighboring allied cortical focus (Graham Brown’s “secondary facilitation”). What is common in all these instances is that antecedent stimulation has so altered the functional condition of the central nervous system (CNS) that the response to subsequent stimulation (i.e. to test stimulation) is changed in the direction of more response. To produce this effect the antecedent stimulation must excite, and we define here as factors for facilitation any changes of the CNS caused by antecedent excitation and causing a fall in threshold, a decrease in latency, an increase in amplitude or any combination of these.
The phenomenon designated “extinction,” discovered in 1934 by the present authors, is a diminution or absence of response on repetition of stimulation of a “motor” focus within an interval longer than that required for facilitation. The latency of a partially extinguished response is markedly greater than of that to test stimulation alone. Subliminal antecedent stimulation of the same focus may yield extinction.(2, 3) What is common in all these is that antecedent stimulation has so altered the functional condition of the CNS that the response to test stimulation is changed in the direction of less response. The antecedent stimulation must excite, and we would define as factors for extinction any changes of the CNS caused by antecedent excitation and causing a rise in threshold, an increase in latency, a decrease in amplitude or any combination of these.
In all that follows one must not confuse extinction—the diminution of motor response resulting from antecedent excitation of the parts of the CNS responsible for that same response—and inhibition, i.e. the diminution of motor response resulting from contemporaneous excitation in parts of the CNS responsible for an antagonistic response. Thus, though extinction and inhibition both result in diminution of motor response, inhibition depends upon reciprocal innervation. extinction does not.
Methods
All experiments were performed on Macaca mulatta monkeys, fully anesthetized with Dial3 (Ciba), 0.45 cc. per kg. bodyweight, half of the dose given intraperitoneally. half intramuscularly.
The duration of the electrical stimulations and the intervals between them were rigidly controlled by the timing-device previously described.(3) The pulses used to stimulate the cortex were either 60∼ through a center-tap transformer grounded at its midpoint4 or those of a Thyratron-stimulator after Schmitt and Schmitt,(4) permitting independent variation of the number of pulses per second (pattern-frequency), and the shape, or duration, of the individual pulses (pulse-frequency) as well as the voltage. These voltages are in this paper given in terms of the readings (in) on a decade voltage-divider (VD) of 10,000 &.#x2126;
The apparatus and electrodes used for the various purposes of the particular groups of experiments will be specified later. Suffice it here to say that for simple stimulation and for recording of the ordinary electrical activity of the various portions of the CNS regular stigmatic Ag-AgCl electrodes were used. In those cases in which “after-potentials” or other slow components were studied special Agar-Ag-AgCl electrodes were used in combination with a D.C. amplifier and cathode ray oscillograph or with a Burr-Lane-Nims microvoltmeter.(5) The pH determinations were performed by a method previously described.(6, 7) The peripheral motor responses were recorded isotonically by tambours either on smoked paper or photographically with the cathode ray oscillograms.(8) The Agar-Ag-AgCl electrodes were made for us by Dr. L. F. Nims, who also gave his invaluable cooperation in those experiments in which pH was recorded.
The designation of the various areas of the sensorimotor cortex is that given in a previous paper.(9)
Results
Since both facilitation and extinction are changes in motor response, these can be observed only by comparing the response to test stimulation following antecedent stimulation with the response to test stimulation alone. This comparison presupposes that the physical properties of the test stimulus are constant, which depends upon constancy of the circuit both external to the animal and within the animal. This constancy has been established experimentally with a method previously described,(10) which permits determination of the instantaneous voltage and current through the specimen during stimulation.
A. Necessity of Antecedent Neural Excitation
In order to conclude that differences in response to constant test stimulation result from neural excitation it is necessary to exclude changes due
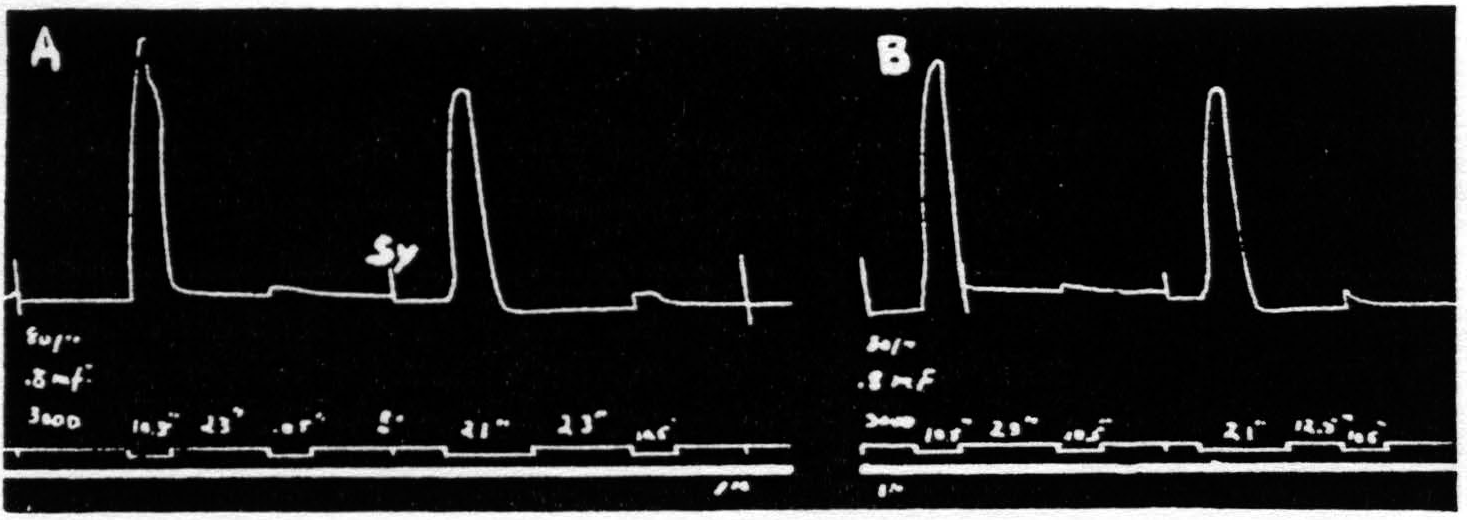
Figure 1. Oct. 22, 1936. Macaca mulatta. Dial-narcosis. Record A shows 2 pairs of stimulations with all parameters the same, except that the antecedent stimulation in the 2nd pair is twice as long as that in the 1st pair. No increase in extinction. Record B repeats record A, except that the interval in the 2nd pair is half that in the 1st pair. Again extinction is not increased. Thus, whether one figures the effective interval as that between stimulations or that between responses, doubling the duration of the antecedent stimulation used here failed to increase extinction. In this and all subsequent figures, “sy” indicates synchronous points.
merely to the passage of the stimulating current. Gross changes of this nature were excluded indeed in experiments using the method above. Furthermore, the findings enumerated below are not explicable in terms of such changes: (i) motor response to cortical stimulation can be facilitated by cutaneous stimulation (Bubnoff and Heidenhain); (ii) prolonged stimulation far enough below threshold does not produce facilitation or extinction; (iii) antecedent stimulation of such a duration that the response begins to decline before the end of stimulation produces as much extinction as does a stimulation twice as long (see Fig. 1); (iv) secondary facilitation, though present from widely separated foci in one subdivision of the sensorimotor cortex, is absent from near foci across the functional boundaries between leg-, arm- and face-subdivisions (see Fig. 2). This type of facilitation must obviously depend upon activity propagated transsynaptically, which is necessarily neural activity.
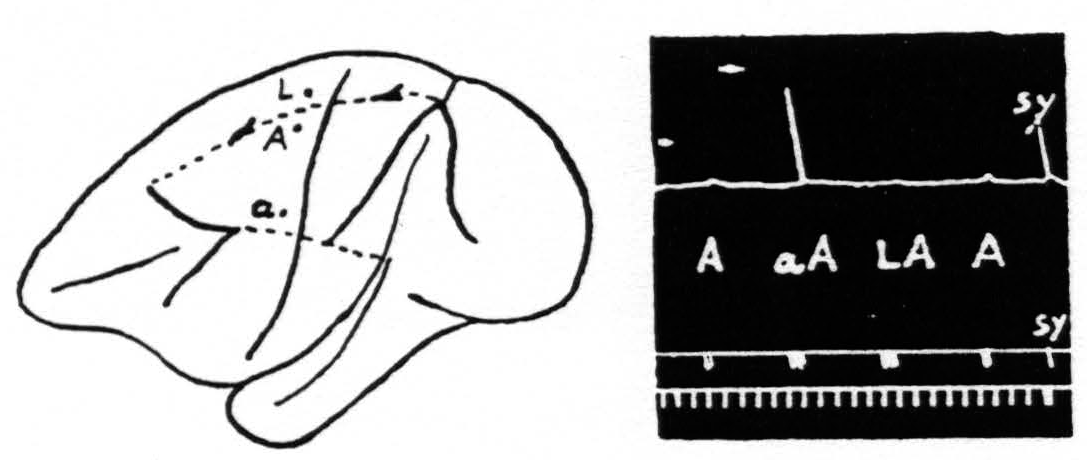
Figure 2. Oct. 27, 1936. Macaca mulatta. Dial-narcosis. This figure shows the existence of secondary facilitation at A from a, 8 mm. apart, and the absence of facilitation across the functional boundary between the leg- and arm-subdivisions, although A and L are only 3 mm. apart.
From the above findings it is clear that observed differences in response, whether facilitation or extinction, cannot be referred to changes produced by mere passage of current of the antecedent stimulation, but must be referred to changes in the CNS resulting from neural excitation elicited by the antecedent stimulation.
B. Multiplicity of Factors
Some justification is required for speaking of factors for facilitation and factors for extinction instead of merely referring the two phenomena to increase and decrease of some single entity such as “excitability.” Scrutiny of the following observations discloses the justification: (i) Facilitation and extinction can coexist.(11, 12) If three equal stimulations are applied to one cortical “motor” focus in such temporal relation that the second and third both fall during the period of extinction induced by the first, but the third falls in the period of facilitation of the second, then the third response shows clear evidence of facilitation, though not as much as when the first stimulation is omitted (see Fig. 3): (ii) Facilitation by one criterion may concur with extinction by another. For example: a decrease in amplitude (extinction) may be associated with a decrease in latency (facilitation) (see Fig. 4); (iii) Facilitation of response to one type of test stimulation and extinction of response to another can be produced by a single type of antecedent stimulation (see Fig. 5).
It is difficult to conceive how this group of observations can be explained on the basis of changes in any one single parameter of nervous activity, e.g. in excitability. Nor is the difficulty solved, so far as we can see, by taking excitability as a variable, i.e. as being different at one time and place in the CNS from what it is at another. Thus we felt compelled to search for several
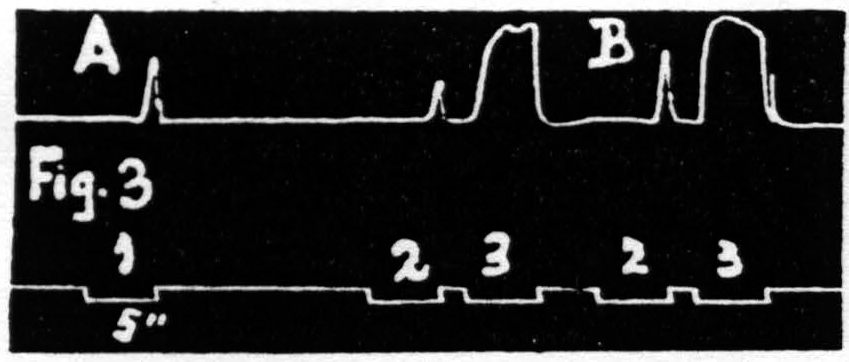
Figure 3. Jan. 20, 1936. Macaca mulatta. Dial-narcosis. In record A three equal stimulations are applied to a “motor” focus. The response to stimulation 2 is extinguished, at this long interval, by the excitation set up by stimulation 1. The response to stimulation 3 is facilitated, at this short interval, by the excitation set up by stimulation 2. Record B shows the facilitation, at this interval, without extinction when stimulation 1 has been omitted.
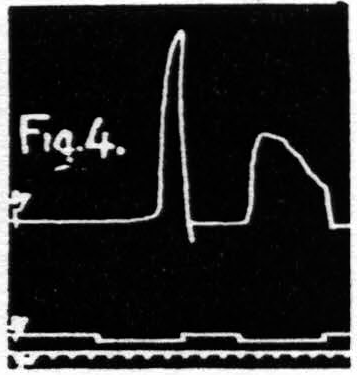
Figure 4. Oct. 16, 1936. The response to the second stimulation of a “motor” focus shows a shorter latency (facilitation) and a smaller amplitude (extinction) than the response to the first stimulation of this focus, i.e. dissociation of latency and amplitude.
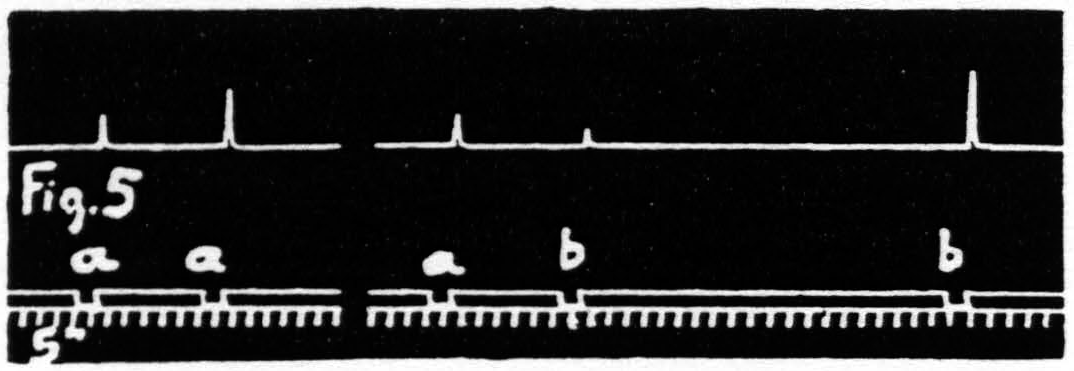
Figure 5. Jan. 31. 1935. Macaca mulatta. Dial-narcosis. Stimulation “a” facilitates response to “a”, “a” extinguishes response to “b”, as seen by comparing it with response to “b” alone.
factors for facilitation and extinction. That these factors had to be conceived as variables having different values at different times and places follows from the results of the following type of experiment, in which four stigmatic Ag-AgCl electrodes,—1, 2, 3 and 4,—are placed 1.5 mm. apart on a given “motor” area (e.g. Arm 4) on a straight line. Their positions have to be such that the primary movements obtained on monopolar stimulation of each of these four foci have an element in common, say extension of the wrist. It is then possible to investigate the effect of antecedent stimulation of focus 1, 2, 3 or 4 upon this common element of the response to test stimulation to focus 1 and thus to ascertain the spatial distribution of facilitation and extinction combined. By changing the interval between antecedent and test stimulation one can thus study the intensity of facilitation and extinction as a function of space (the separation of electrodes) and time, or by
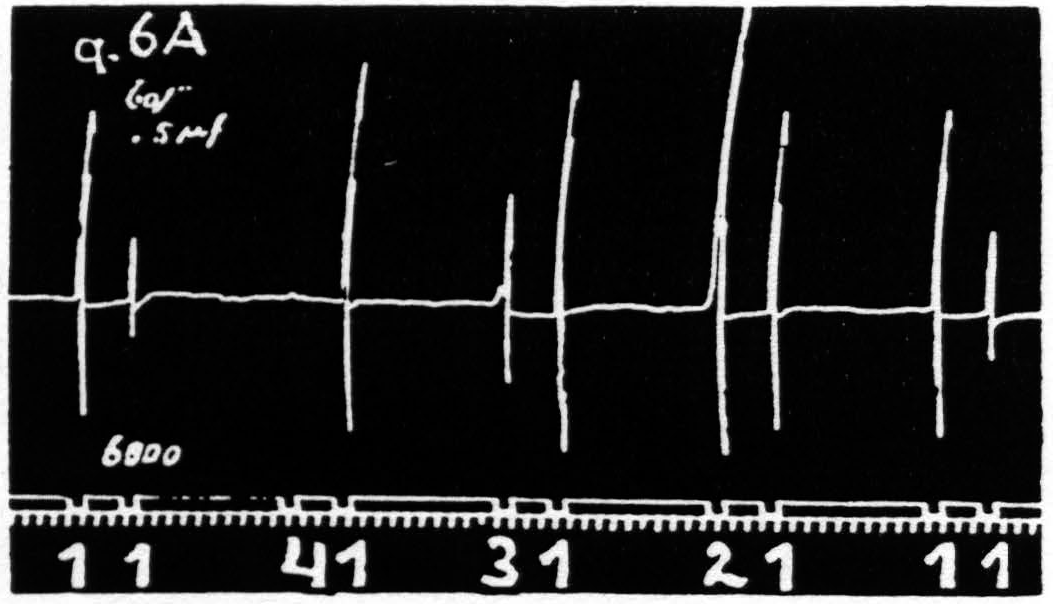
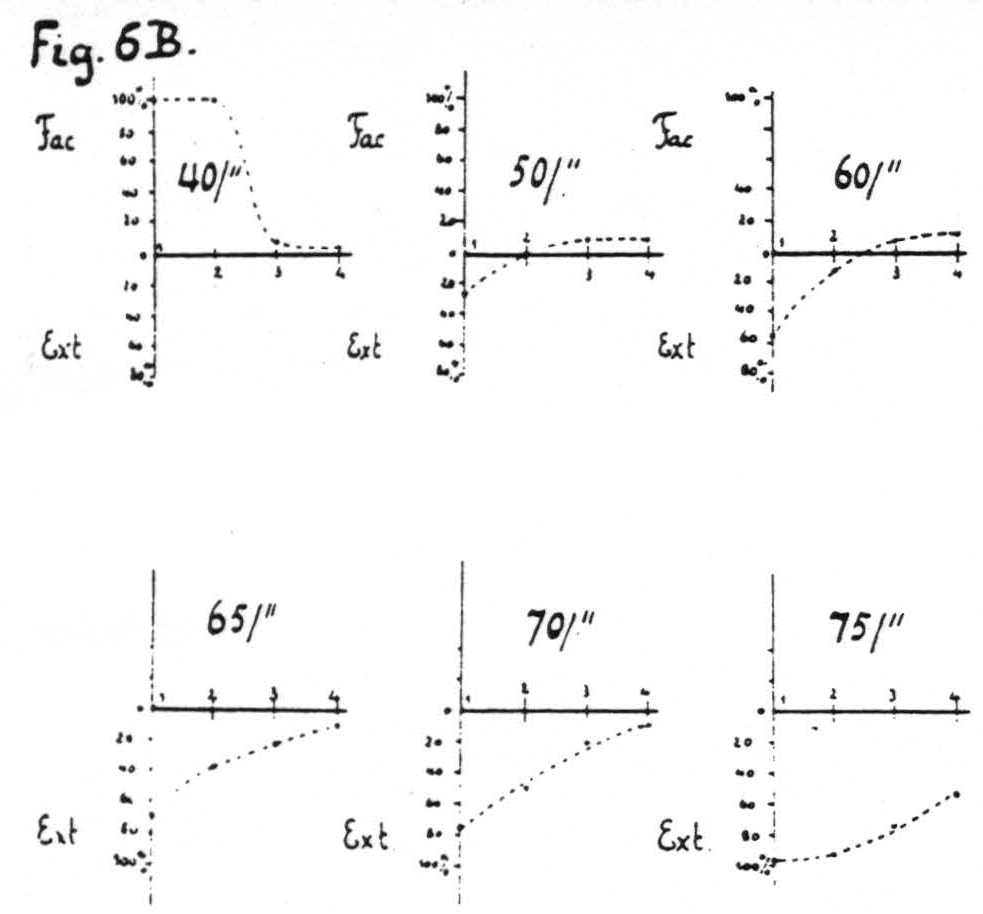
Figure 6a. Feb. 21, 1935. Macaca mulatta. Dial-narcosis. Four stigmatic Ag-AgCl electrodes, 1.5 mm. apart, are so placed on a straight line on the “motor” arm area that the responses to stimulation of each of these foci have an element, extension of the wrist, in common. Record shows extinction from 1 on 1, equalization of response from 2 on 1, facilitation from 3 and from 4 on 1.
Figure 6b. Same arrangement of electrodes as in Fig. 6a gives the average percentage change in size of many responses to test stimulation at focus 1 induced by antecedent stimulation at foci 1, 2, 3 and 4 respectively for various pattern-frequencies; all othe7 parameters of stimulation and the interval between members of a pair being held constant throughout. At low frequencies pure facilitation in the vicinity of focus 1, at intermediate pattern-frequencies pure extinction around focus 1, facilitation at some distance (foci 3 and 4), at higher frequencies extinction from all foci, increasing as the frequency increases.
changing any of the parameters of stimulation, at constant interval, as a function of the separation of electrodes and the parameter varied. In the experiments of Fig. 6 the stimulations were at constant interval and the parameter varied was the pattern-frequency. On stimulation with lower pattern-frequencies facilitation was found greatest at focus 1 from focus 1, decreasing so as to be least at focus 1 from focus 4; with higher pattern-frequencies the same obtained for extinction. With intermediate pattern-frequencies extinction was found at focus 1 upon antecedent stimulation of near foci and facilitation upon antecedent stimulation of more distant foci. Figure 6A is an excerpt from a record with an intermediate pattern-frequency (60 per sec.); Fig. 6B shows diagrammatically this spatial distribution of facilitation and extinction combined as a function of the pattern-frequency of stimulation.
C. Factors for Facilitation and Extinction
The foregoing experiments have indicated the necessity of considering factors for facilitation and extinction, differing in kind and distribution. Inasmuch as these factors are functional changes in the CNS they can be disclosed only by examining what is going on within it. Since only changes in threshold and changes in activity can affect the response to test stimulation and since threshold and activity can both be affected by antecedent activity both must be investigated. Changes in the activity of any part of the CNS can be studied by recording the electrical activity of that part; changes in threshold of any part can be studied by determining the minimal electrical stimulation which produces an electrical response at that site.
It is essential to emphasize that, although these significant variables can be studied separately, they are inevitably interrelated in the living CNS. With impulses always impinging upon the part of the CNS under investigation a fall of threshold permits increased activity, increase of activity produces a rise of threshold. But that is not all. In peripheral nerve, changes in threshold have long been known to be associated with “after-potentials" and with changes in ion-concentration, notably changes in pH. Therefore, in the following group of experiments the changes in activity and threshold were investigated by recording the electrical activity of various parts of the CNS, the threshold of the cortex, its “slow potentials"* and its pH before and after such electrical stimulation of the cortex as was shown to give facilitation or extinction of motor response to a particular test stimulation at a certain focus and after a given interval. It should be realized that in order to ascertain the altered electrical activity, “slow potentials” and pH of the cortex or other parts of the CNS at the time when the test stimulation would fall, this stimulation has to be omitted. Therefore, a separate, parallel experiment with antecedent and test stimulation has always to be made to ascertain whether, where and when facilitation or extinction obtains. Without both experiments it is impossible to infer what and how distributed in space and time are the functional changes underlying facilitation and extinction.
One of the conclusions from the experiments represented in Fig. 6 was that one obtained facilitation or extinction depending upon the distance of the locus of the test stimulation from that of the antecedent stimulation. This means that one has to investigate the changes mentioned above in the immediate neighborhood of stimulation, at foci in the vicinity, at distant foci in the cortex and finally at other levels of the CNS.
1. Changes in electrical activity
a. In the immediate neighborhood of cortical stimulation. Electrical stimulation of the cortex such as to produce profound extinction results in a temporary diminution or silencing of the electrical activity at the site and in its immediate neighborhood.(13) For optimal localization of stimulation and recording of the activity at the site of stimulation it is necessary to use for both a single pair of concentric electrodes. We have employed concentric Agar-Ag-AgCl electrodes, the central electrode ending in a glass capillary 0.1 to 0.3 mm. in diameter and the circumferential circular electrode, between concentric glass tubes, about 6 mm. in diameter and 1 mm. wide.2 Figure 7 shows a cathode ray oscillogram of the electrical activity of the cortex before and immediately following extinguishing stimulation under these conditions, the D.C. amplifier being disconnected from the specimen during stimulation. It will be noted that the electrical cortical activity following this extinguishing stimulation, although subthreshold in regard to motor response, is greatly reduced. With electrical stimulation apt to produce motor after-discharge (long pulses, e.g. 60 ∼ ) one is likely to find immediately after the stimulation, even when subthreshold in regard to motor response, a short period of electrical after-discharge followed by a longer period of diminished electrical activity of the cortex. Figure 8 illustrates this statement. The form of the spikes in the discharge indicates (as will be discussed later) that it started in the cortex nearer the central electrode and ended under the circumferential electrode, so that at this stage facilitation was present around the area of extinction under the central electrode (a situation comparable to graphs 2 and 3 of Fig. 6B), whereas a little later the cortex underneath both electrodes had passed over into the stage associated with extinction.
b. In neighboring foci. For recording of changes in electrical activity several millimeters away from the locus of stimulation but still in the cytoarchitectonic area of the same subdivision of the sensorimotor cortex two pairs of electrodes can be used, thus obviating difficulties of interpretation. The phases of afterdischarge and diminution of activity, corresponding to facilitation and extinction respectively, are more prolonged and often more


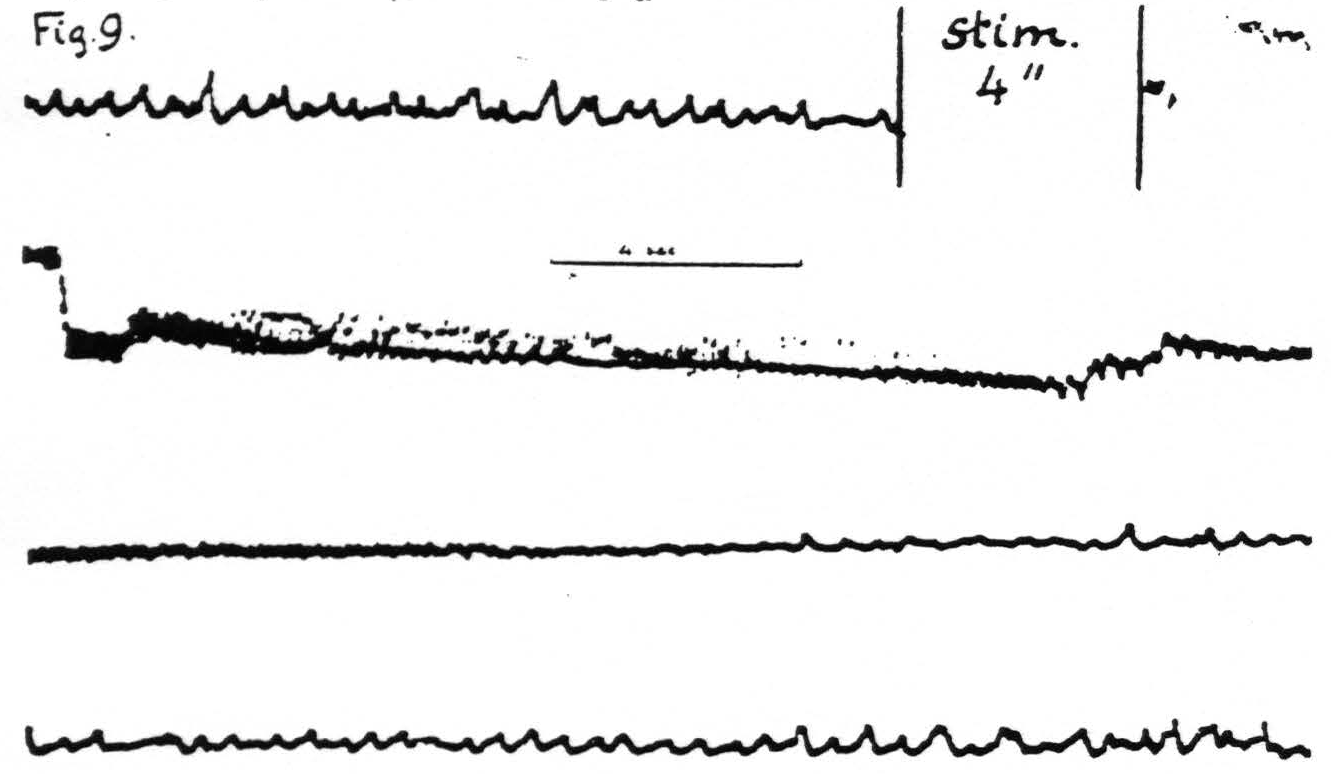
Figure 7. Jan. 18. 1936. Macaca mulatta. Dial-narcosis. Cathode ray oscillogram of electrical activity of a focus of the “motor” arm area before and after electrical stimulation of this focus. Concentric Agar-Ag-AgCl electrodes used for stimulation and pick-up from a focus of motor arm area. Extinguishing subthreshold stimulation results in a very short-lived negative voltage drift followed by a positive drift and initial diminution of electrical activity of this focus. Lower heavy white line shows period of stimulation, upper heavy white line shows absence of peripheral motor response.
Figure 8. Jan. 17, 1936. Same arrangement of stimulation and pick-up electrodes as in Fig. 7. This extinguishing stimulation (60∼), known to give motor after-discharge at or near threshold voltages, produced an initial electrical after-discharge followed by diminution of electrical activity and gradual return to normal.
Figure 9. Jan. 28, 1936. Macaca mulatta. Dial-narcosis. Cathode ray oscillogram of electrical activity of cortex before and after electrical stimulation. In this experiment two stimulating stigmatic Ag-AgCI electrodes were placed on dorsal portion of “motor” leg area (L.4) and parallel to them, 3.5 mm. distant, two similar pick-up electrodes on the ventral portion of the same area, thus minimizing voltage drifts. The subthreshold stimulation resulted in a marked prolonged electrical after-discharge followed by diminution of electrical activity extinction) with gradual return of it to normal. Continuous record.
pronounced. An example is supplied in Fig. 9 in which it will be seen that, following the electrical stimulation of a focus of L.4, the electrical activity of a focus of the same area 3.5 mm. distant is at first greatly increased—electrical after-discharge—then diminished to return to normal about a minute after stimulation.
c. In more remote parts of the cortex. Under this heading comes the description of the changes in electrical activity in various cytoarchitectonic
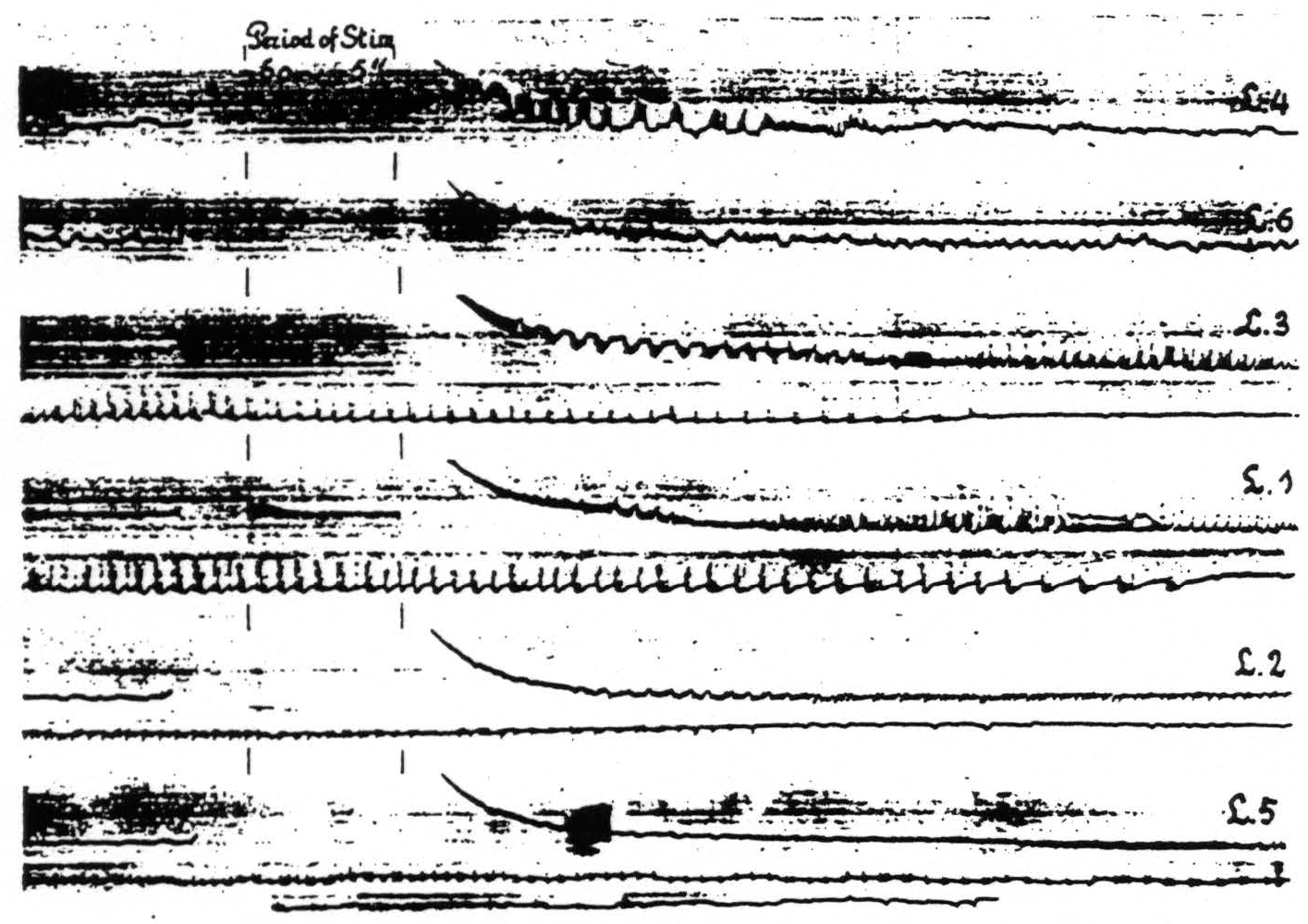
Figure 10. April 23, 1936. Macaca mulatta. Dial-narcosis. Cathode ray oscillograms of electrical activity of various foci of the leg-subdivision of the sensorimotor cortex. In each of the records of this figure L.4 was stimulated (60∼, 5 sec.) with bipolar Ag-AgCl electrodes and the electrical activity recorded with similar electrodes from the named areas successively. For further comments see text.
areas of one subdivision upon stimulation anywhere within this subdivision. With one pair of stimulating electrodes always on, let us say, L.4 the electrical activity of another locus in this area and of associated areas of this subdivision (L.6, L.3, L.1, L.2 and L.5) was recorded successively before and after stimulation. Figure 10 shows the changes in electrical activity in these various leg areas following stimulation of L.4. In the particular experiment to which Fig. 10 relates the pick-up electrodes were so arranged that those in the leg areas were successively connected to the grid of the amplifier, the cathode being simultaneously connected to an electrode in the corresponding area of the arm-subdivision. This arrangement allowed one to interpret the sign of the recorded deflections in terms of the site and direction of propagation of the electrical activity in the leg-subdivision, since the activity of the arm-subdivision was not influenced by the stimulation of L.4.
In an experiment of this kind with a D.C. amplifier and intended to record slow “D.C. potentials,” the usual procedure of placing one of the electrodes as a reference-electrode on a killed portion of the cortex is inappropriate on account of the spurious slow potentials produced.
Examination of Fig. 10 reveals the following: a. subliminal stimulation of L.4 results in electrical after-discharge not only in L.4, but also in L.3, L.1. L.2 and L.5. not in L.6—a distribution of hyperactivity in entire harmony with the functional organization of the cortex as revealed by local strychninization;(9) b. comparison of the pictures of after-discharge in L.4 and L.3 shows that the sign of the rapid potential fluctuations in L.4 is always in the same direction (upwards =negative), whereas the fluctuations in L.3 are initially of the opposite sign, then suddenly reverse. So far as we can see there is only one possible interpretation, compatible with our analysis of the strychnine-spike,(14) namely that negative swings and negative spikes express activity originating in the area itself with the superficial layers leading, whereas the positive swings with positive spikes indicate activity with the deeper layers leading. This interpretation is furthermore in harmony with the findings and interpretations of Bishop and collaborators,(15, 16, 17, 18) of Marshall, Woolsey and Bard(19, 20) and of Adrian.(21) This indicates that L.4. which was stimulated electrically, is itself in active discharge immediately after this stimulation. whereas L.3 at first receives impulses from L.4 resulting only after more than 10 sec. in an active discharge of the area itself. Essentially the same can be seen in the other records of the postcentral areas and it is of interest to note that the active phase of the discharge begins later and ends later the more remote the area from L.4.
In these experiments sub C 1, a, b and c the period of electrical after-discharge is regularly followed by a period of electrical inactivity, as can be seen from Figs. 8 and 9. This phenomenon is not obvious in Fig. 10 because the animal was deeply narcotized and the amplification used was purposely small to insure full recording of the spikes of the after-discharge.
d. In distant parts of the CNS. Associated changes in electrical activity can be observed in other portions of the CNS, e.g. the spinal cord.(13) In Fig. 11 is shown the electrical after-discharge in the white matter of the lumbar enlargement (ventrolateral column) of a monkey’s cord following electrical stimulation of a focus of the cortex of L.4. These four records were made with increasing voltage of stimulation, the last being just supraliminal. Figure 12 is a record of the electrical activity of the grey matter (ventral horn) of the cervical enlargement of the cord before and after subliminal electrical stimulation of a cortical focus of A.4. Comparison of these two typical records shows that while the after-discharge in the white matter resembles that obtainable from the cortex, the picture from the grey matter differs, in that
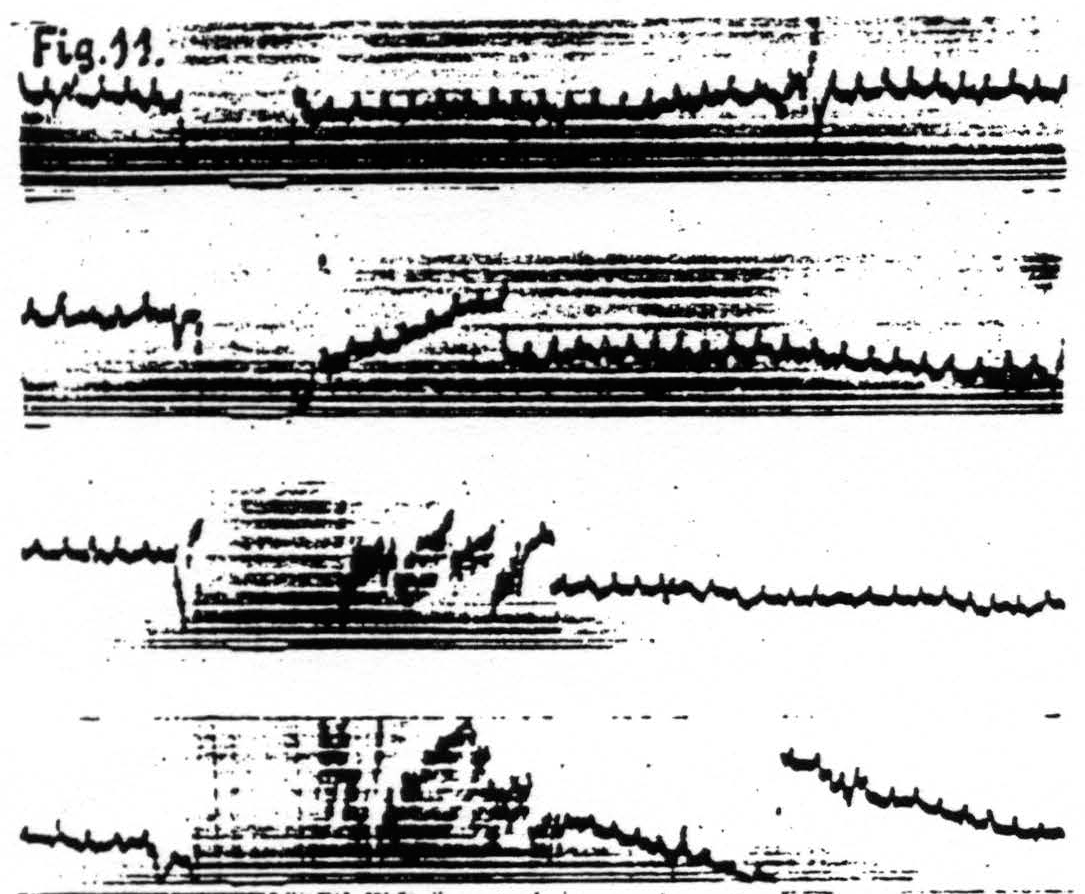
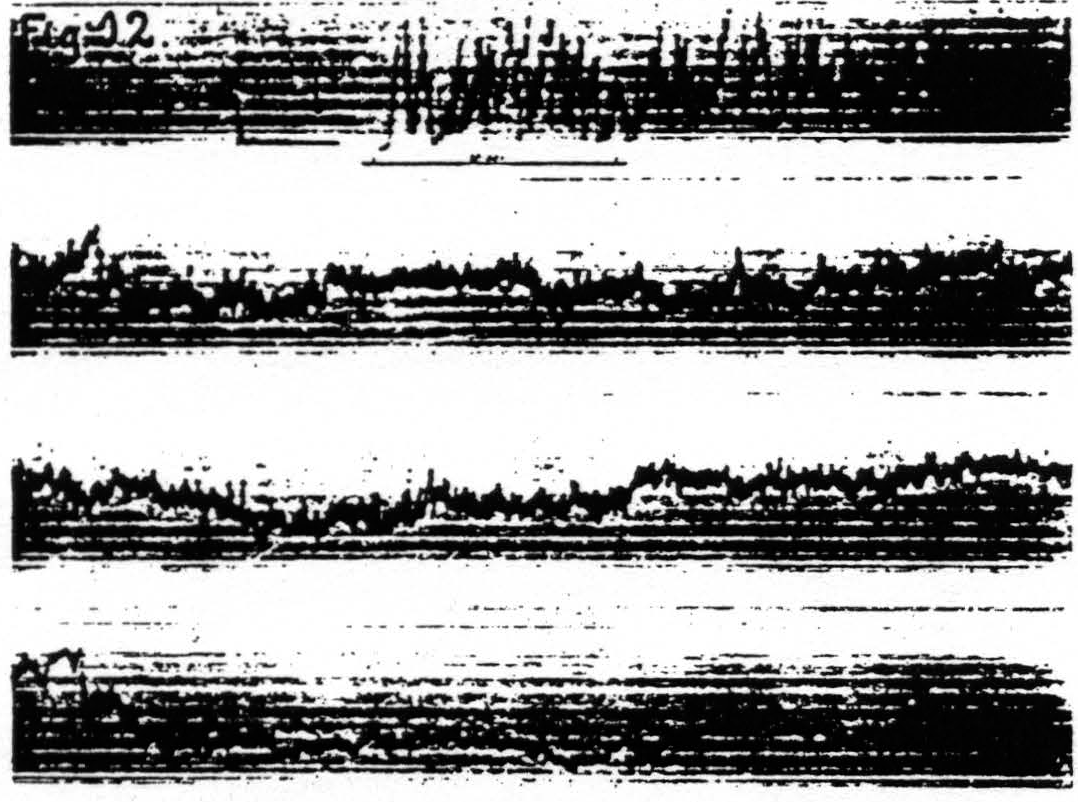
Figure 11. Jan. 29, 1936. Macaca mulatta. Dial-narcosis. Cathode ray oscillograms of electrical activity of ventrolateral column of lumbar enlargement of spinal cord before and after electrical stimulation (60 ∼, 4 sec.) of contralateral motor leg area (L.4). Strength of stimulation in record 1 = 2000, in record 2 = 3000, in record 3 = 4000, in record 4 = 6000. The last stimulation was just supraliminal (see upper heavy white line in 4). Note voltage drifts so great as to require balancing of spot on screen of cathode ray oscillograph. Lower heavy white line in all records shows period of stimulation.
Figure 12. Jan. 20, 1936. Macaca mulatta. Deep Dial-narcosis. Cathode ray oscillogram of electrical activity of ventral horn of eighth cervical segment of spinal cord before and after bipolar subthreshold stimulation of focus of contralateral “motor "arm" area (A.4). Note the prolonged electrical after-discharge in grey matter of cord. Under this deep narcosis extinction lasted more than 5 min., less than 10 min. (Continuous record.)
it is more irregular and much more prolonged. Once these changes in electrical activity following one period of stimulation of the cortex were known, their relation to facilitation and extinction could be investigated by applying at an appropriate interval a second. or test, stimulation. During the early stages of after-discharge in a “motor” area (e.g. A.4) induced by stimulation of this area itself or of a remote related area (e.g. A.5) test stimulation to this “motor” area (A.4) results in a facilitated motor response. whereas during the later stages of after-discharge this test stimulation elicits a smaller or no motor response (extinction) and even frequently terminates the existing after-discharge. The motor response to test stimulation applied during the period of electrical inactivity of the cortex following an electrical after-discharge is always extinguished.
2. Slow voltage drifts
When records of the electrical activity of the cortex before and after stimulation are taken with a direct-coupled amplifier the site of stimulation is seen to be negative at the end of stimulation and to become slowly positive with respect to a distant focus. These slow drifts appear with almost any type of electrode. With due precautions as to the type of electrodes and circuits used for pick-up and stimulation these changes can be shown to be not artifacts but physiological phenomena. To observe these changes as precisely as possible at the site of stimulation requires again that a single pair of concentric electrodes be used both for stimulation and pick-up. Obviously for such an experiment the electrodes must be non-polarizable: as such we used the concentric Agar-Ag-AgCl electrodes mentioned above, which in practice were found to be good to some 4 or 5µV. It was with these electrodes that the record of Fig. 7, showing the negative (up) and positive (down) slow drifts at the site of stimulation, was obtained. For investigation of slow drifts at foci other than that stimulated separate pairs of electrodes for stimulation and pick-up can be used. This in itself obviates one of the possible sources of artifact, i.e. alteration of the pick-up electrodes by passage of the stimulating current. Moreover. one can use for stimulation a transformer, with a center-tap grounded and permanently connected to the cathode of the amplifier. For foci near the site of stimulation one of the pick-up electrodes, to wit, the “live” electrode, can be placed half-way between the two stimulating electrodes and connected to the grid of the amplifier, except during stimulation when it must be disconnected. The reference-electrode, placed on a distant unrelated cortical focus (i.e. one not affected by the stimulation) is connected permanently with the cathode of the amplifier, ground and the center-tap of the transformer. This circuit is so symmetrical as to be practically free of D.C. artifacts, except for a slight shift produced by rectification of the A.C.-current in the transformer. The slow voltage drifts recorded in this way are essentially similar to those already described: they are somewhat larger and more complicated by action potentials due to the greater separation of the electrodes. Moreover inasmuch as the “live” lead is
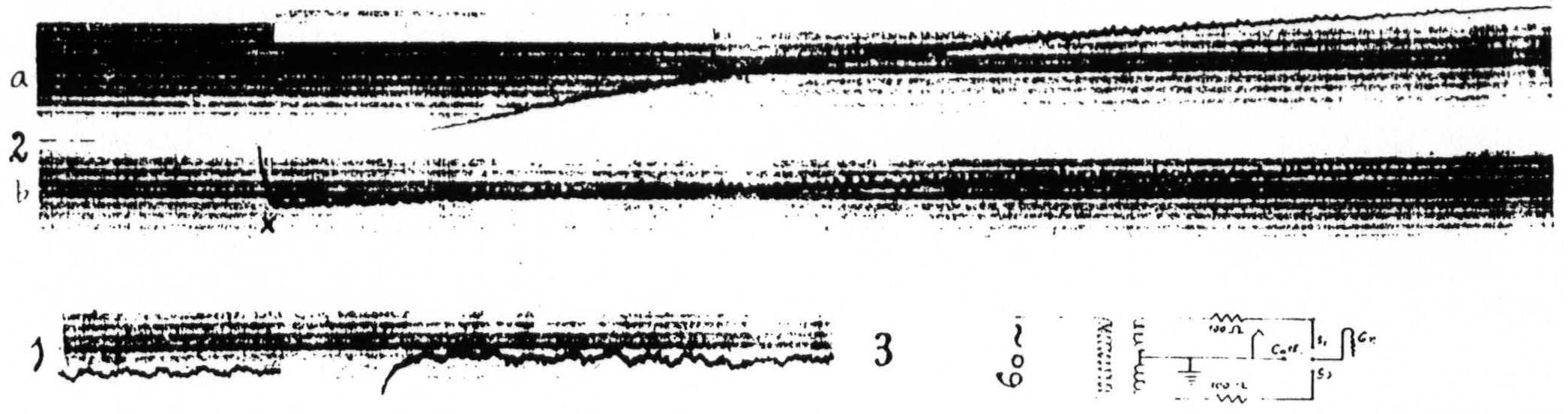
Figure 13. April 16, 1936. Macaca mulatta. Dial-narcosis. Record 3 shows arrangement of four electrodes (Agar-Ag-AgCl) on cortex and their connections to the center-tap transformer, and to the cathode ground and grid of the amplifier. Record 1 shows the electrical activity and the small and short-lived negative (down) and positive (up) voltage drifts following liminal stimulation (4 sec., 60∼) of a focus of the motor arm area (A.4) in the lightly narcotized animal. Record 2 shows the marked and prolonged negative and positive voltage drifts following liminal stimulation (4 sec., 60∼) of the same focus when the animal was deeply narcotized. Sections a and b of record 2 continuous. The insert in this record shows the interval at which equalization of threshold response occurred in this stage of narensis. x — balancing of spot on screen.
not exactly at the site of stimulation, they never show the complete inactivity (extinction) revealed with a single pair of concentric electrodes. Figure 13(16) shows diagrammatically the connections of the center-tap transformer and the amplifier to the four electrodes on the cortex. Figure 13(21) and 13(15) were obtained on the same animal, in both cases with liminal 60∼ stimulation. Figure 13(21) was obtained in the lightly narcotized monkey when facilitation lasted only a few seconds, whereas Fig. 13(15) was recorded under deep narcosis. It will be seen that in the latter case the stimulation is followed by a very marked negativity (downward) succeeded by a long lasting positivity (upward), so great as to require balancing of the spot on the screen of the cathode ray oscillograph at the point
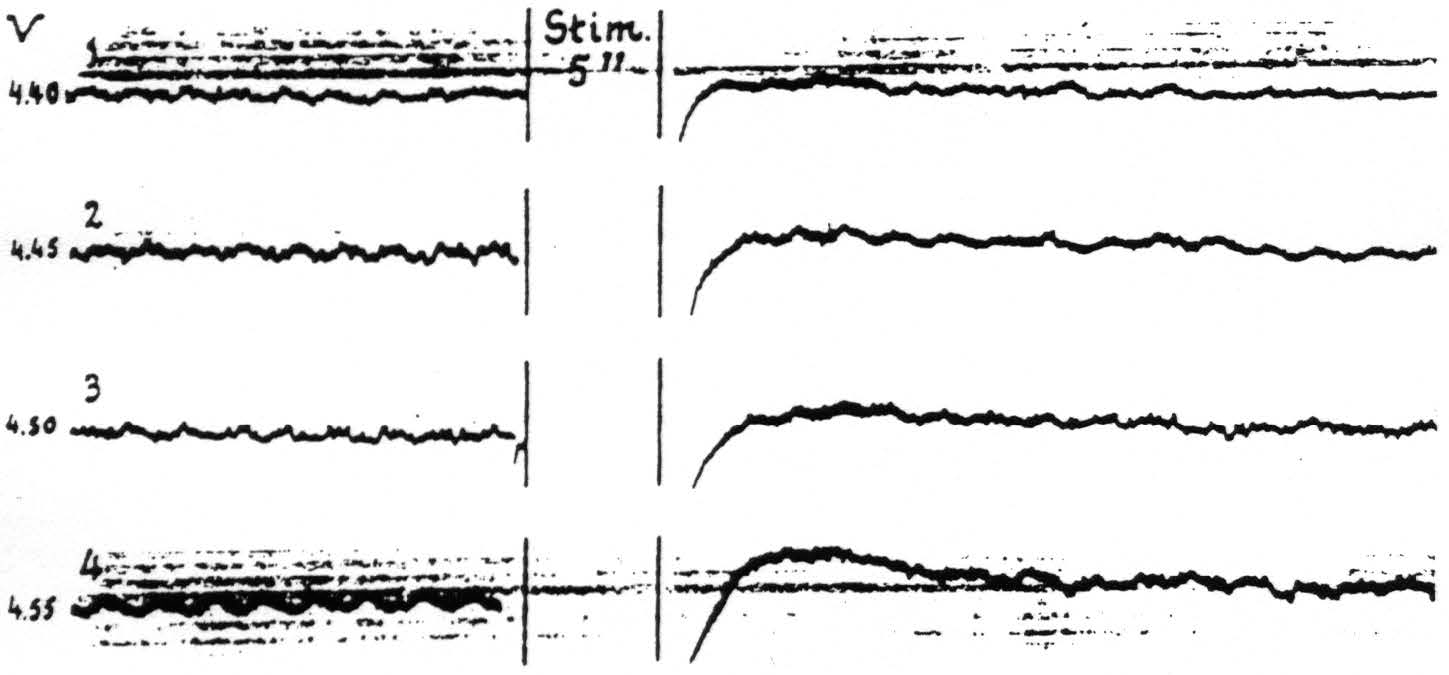
Figure 14. April 15, 1936. Macaca mulatta. Light Dial-narcosis. These cathode ray oscillograms show the gradual increase of electrical after-discharge and negative and positive voltage drifts with increase of stimulation of a focus of A.4. All stimulations (4 sec., 60∼) the same except for voltage. Arrangement of electrodes as in Fig. 13. With these strengths of stimulation facilitation lasted as long as the periods of fast action potentials of these records. Voltages (V) at left of records.
marked x. On Fig. 13(15) is an insert, from a record of a parallel experiment and taken with the same speed of paper, showing the interval at which repetition of the stimulation yields equal motor response. It will be observed that this happens at a time between the negativity and positivity when the voltage is practically zero. At all intervals shorter than this “equalization” interval facilitation of motor response was encountered; outside this interval for a period of many minutes, i.e. much longer than the record represents, total extinction of motor response obtained. Thus “negativity” is associated with facilitation, “positivity” with extinction. These voltage drifts are sufficiently prolonged to admit recording with a microvoltmeters and an ordinary sensitive galvanometer, and by this method their sign and magnitude are found to be as indicated. Since the period of such a galvanometer is relatively long it distorts the time-relations and for this reason no such record is presented.
Such a simple association of these voltage drifts with facilitation and extinction is found only when electrical after-discharge is avoided by proper selection of the stimulation. With after-discharge, which during its stage of rapid discharge has been shown above to be a factor for facilitation, the period for facilitation is prolonged into the period of positivity. In the particular experiment of Fig. 14 wherein the after-discharge had only a fast phase, the facilitation lasted as long as the after-discharge, whereafter extinction, even to stronger test stimulation, ensued. Figure 14 demonstrates the changes in electrical activity and the slow drifts following four stimulations of a focus of A.4 at various voltages through capillary Agar-Ag-AgCl electrodes of high resistance. It will be seen that with gradual increase of the stimulating voltage the electrical after-discharge and associated drifts become more pronounced. In all cases there was a motor after-discharge of very small amplitude, though to the first two stimulations (records 1 and 2) there was no visible primary response.
It should be mentioned here that when an electrical after-discharge is propagated into a remote area related to the one stimulated, this remote area at first “climbs” negative, and then, as it begins to discharge actively, “climbs” positive in respect to an unaffected reference-area, and when electrical inactivity ensues it is and remains for some time on the positive side. This means that the activity of a given area has the same effect on its slow voltage drifts regardless of how this activity was initiated, i.e. whether by direct electrical stimulation or by a disturbance propagated into it transsynaptically.
3. Changes in pH of the cortex
It has been shown so far that facilitation is associated with increased electrical activity and a negative voltage drift, extinction with decreased electrical activity and a positive voltage drift. These factors are sufficient to account for the period of facilitation, the time of equalization of motor response and the beginning of extinction. It was found, however, that the positive voltage drift disappeared before the electrical activity of the cortical focus returned and before extinction had passed off. Investigation of the latter part of the period of extinction showed that the end of extinction coincided with the return of normal electrical activity. The question then arose, what factor underlies the terminal portion of the period of electrical inactivity and associated extinction? Obviously this must be a change in threshold. Several old and new observations had pointed toward the pH as a significant variable in determining the activity of the nervous system, more particularly in determining the threshold of isolated nerve. Moreover, it was only reasonable to assume that the increased activity of the cortical cells stimulated would eventually lead to an increased production of acid metabolites. These considerations themselves were sufficient to necessitate pH-determinations of the cortex under various conditions of activity. Fortunately this demand could be met, since the glass-electrode technique of pH-determinations had been developed sufficiently by Nims to be applicable to determinations in vivo and had been already used by him in collaborations with the authors in investigations on the effects of intravenous injections of acids, alkali and changes in ventilation on the pH of the cortex and the corresponding changes of its threshold.(6, 7) In the second paper cited it was stated that a change of pH of the cortex from 7.3 to 7.5, however induced, resulted in a decrease of more than 25 per cent of the voltage required to initiate a minimal electrical after-discharge, and that a decrease of pH to 7.1 raised the threshold much more than proportionally. In the same paper will be found evidence to the effect that hyperactivity of the cortex. whether due directly to electrical stimulation or to an after-discharge propagated into an area investigated, causes a large decrease of pH which cannot be referred to anything but the increased activity of the nerve cells involved, and, finally, that this “acidity” persists as long as the prolonged diminution in electrical activity of the cortex and its concomitant rise in threshold(7) (pp. 286 and 287). In Fig. 7 of that paper an initial alkaline wave, beginning during stimulation, will be seen. At that time we were not certain that this wave might not be an artifact, although it was constantly present with all effective stimulations, regardless of the distance of the pH electrodes from the stimulating electrodes and notwithstanding all possible precautions to make the stimulating current symmetrical and the stimulating and recording circuits as independent as the requirements of the experiment permit. It was not until we had evidence of the association of an alkaline wave in the cortex with the rapid phase of a central electrical discharge, induced by convulsant drugs in the curarized animal,(22) that we were convinced of the significance of the observed initial alkalinity. Since this alkalinity coincides with the early part of the period of negative voltage drift it is impossible to observe the influence of this alkalinity by itself upon cortical threshold and motor response; one can only infer from the observed effect of cortical alkalinity otherwise induced that it must give a fall in threshold of all nerve cells in the “alkaline region” and so operate as a factor for facilitation.
Figure 15 presents diagrammatically the relation of facilitation and extinction of motor response induced by 60∼ stimulation of a “motor” focus to the corresponding changes in electrical activity, voltage drifts and pH _at the site of stimulation.
To summarize the results of the experiment sub C one can say 1. that increased activity, negative voltage drift and alkalinity operate as factors for facilitation; 2. that decreased activity, positive voltage drift and acidity operate as factors for extinction. The distribution of these factors in the CNS, both as regards time and place, is determined by the propagation of neural impulses from the site of initial stimulation, the increased activity in remote areas determining in them slow voltage drifts and changes in pH.
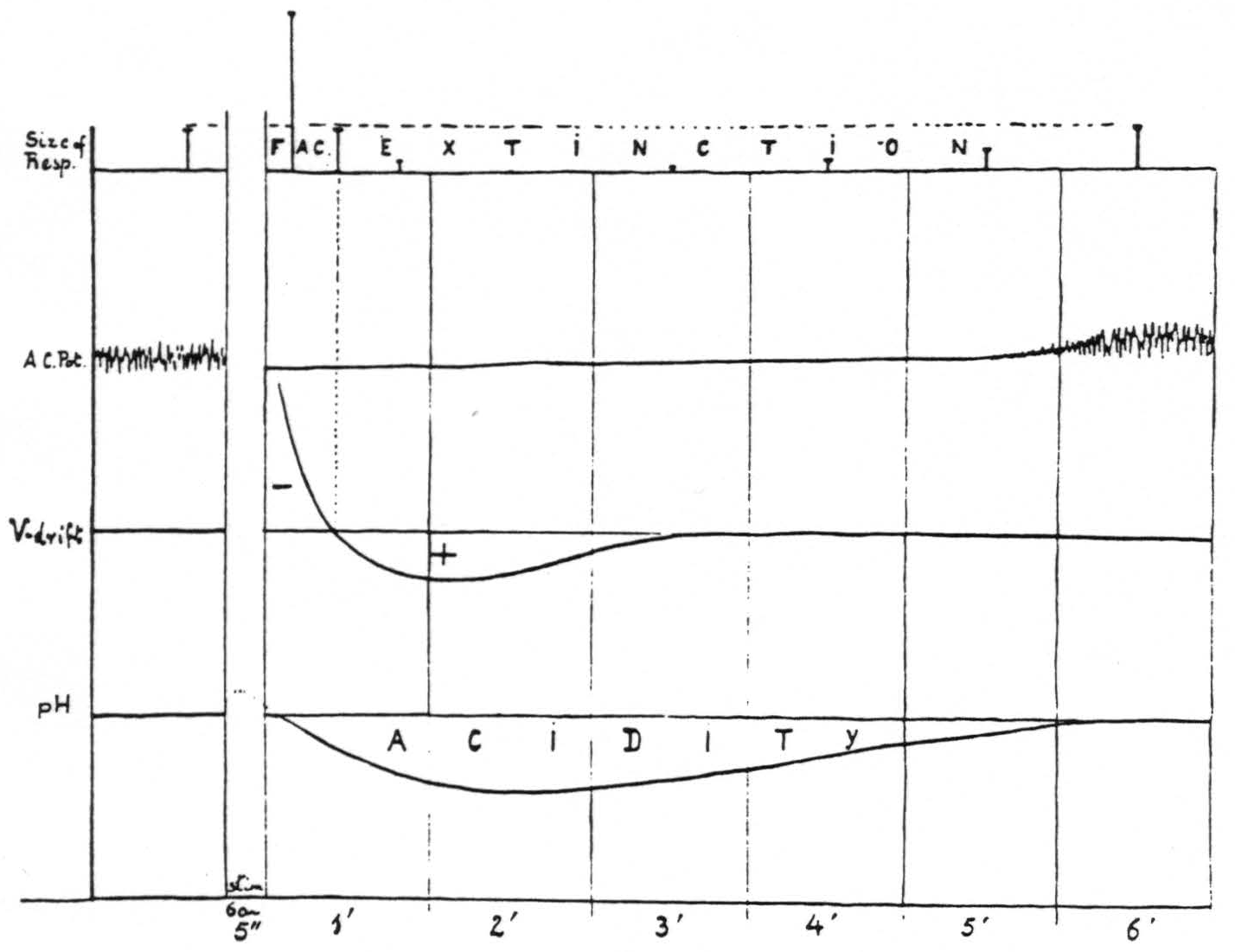
Figure 15. Diagram of correlation of facilitation and extinction with changes in electrical activity, voltage drifts and pH at the site of stimulation.
D. Mode of Action of Factors for Facilitation and Extinction
From general considerations of a host of observations, old and new, of many investigators it seems permissible to infer that the mode of action of these three kinds of factors is as follows: 1. changes in activity as such alter the amount of summation to be expected at subsequent synapses; 2. slow voltage drifts are either the electrical manifestation of changes in temporo-spatially dispersed activity (i.e. action potentials which overlap and sum to give a smooth contour) or changes in voltage of cells that have been discharged (i.e. “after-potentials”) or a mixture of these; 3. changes in pH involve all nervous structures lying within the region of altered pH, and thus involve cells that have not yet been discharged. These implications are of direct interest in connection with many of the problems pertaining to facilitation and extinction. First let us consider the dissociation of changes in latency and in amplitude, as exemplified in Fig. 4, where decrease in latency (facilitation) is associated with decrease in amplitude (extinction). To account for the decrease in latency of a response one must assume that less summation is required, i.e. that there is a fall in threshold to repetitive stimulation, whereas to account for the decrease in amplitude one must assume that fewer nerve cells are capable of responding.
1. Relation of latency and threshold
If the first of these assumptions is correct one must expect that stimulations giving facilitation with decrease in latency but with little or no change in amplitude will produce a marked fall in threshold of the focus under investigation. That this is so can be seen in Fig. 16. In this particular experiment the threshold was slightly below 4300, and 4200 was definitely subthreshold, yet the response to it as test stimulation was as large as the response to the antecedent stimulation at 4600. Even test stimulation at 3500, i.e. following a stimulation at 4600, brought in a just visible response. Figure 16A, therefore, shows that some cells of the nervous structures involved
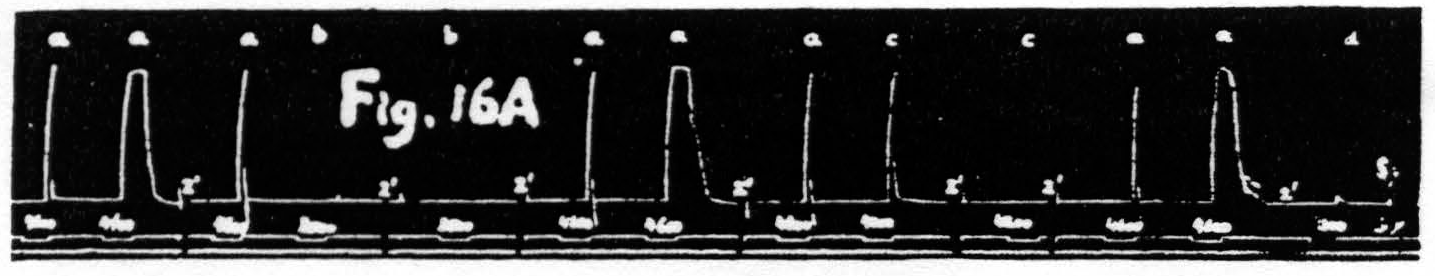
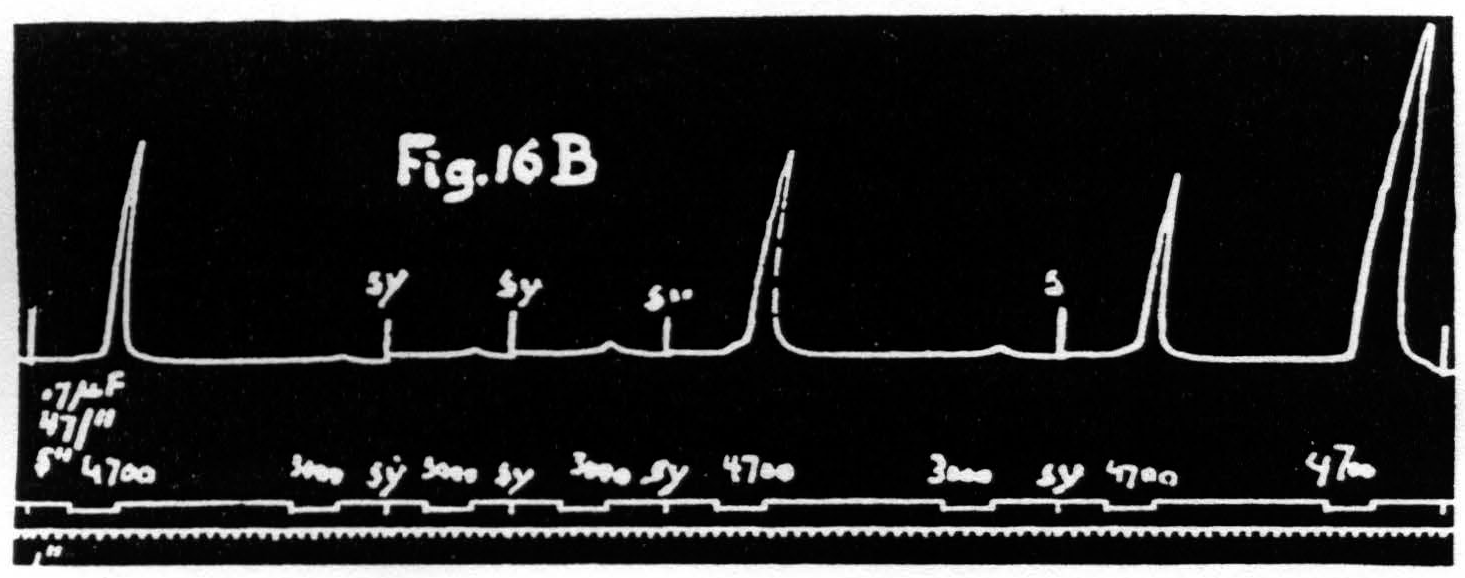
Figure 16a. Dec. 1, 1938. Macaca mulatta. Dial-narcosis. This figure shows that facilitation as judged by decrease of latency is associated with a marked fall of the voltage threshold.
Figure 16b. March 10, 1939. Macaca mulatta. Dial-narcosis. This figure shows constancy of threshold with constancy of latency accompanied by marked increase of amplitude (see last two responses).
have been rendered excitable to voltages formerly far subthreshold. It is even possible to dissociate latency and amplitude in the opposite direction, i.e. to obtain facilitation as judged by increase of amplitude without change of latency or even with a slight increase of latency (see Fig. 16B). Under these conditions there is no obvious change or a slight rise in voltage-threshold. Taken together these findings show that latency and voltage-threshold are covariant regardless of amplitude. Amplitude of response must, therefore, depend upon some factor other than threshold; this must be a change in activity available for summation.
2. Dissociation of latency and amplitude of motor response
The second assumption, namely that during extinction, as judged by decreased amplitude, fewer cells are capable of responding, implies that these cells have passed over into a period of extinction, and this, in turn, that with pairs of equal stimulation the amplitude should decrease with increase of interval because cells previously active or in the period of lowered threshold (negative voltage drift) will now have passed into the period of raised threshold (positive voltage drift), even when the decrease of latency is not yet materially affected by the increase of interval. That this is the case is seen in Fig. 17. This figure shows a series of eight pairs of equal stimulations with gradually increasing intervals and so exhibits the latency and amplitude of the second response as a function of the interval. It will be seen that the latencies of the second responses are smaller than those of the first in graphs a, b, c and d, equal in e, and greater in f, g and h, though the amplitude of the second responses is decreased in all graphs, i.e. the amplitude falls off earlier than the decrease in latency.
It should be noted that between each graph of Fig. 17 there was a pause of 2 minutes. The variations in size of the first responses are the expression of the waves of cortical excitability previously described.(23, 24)
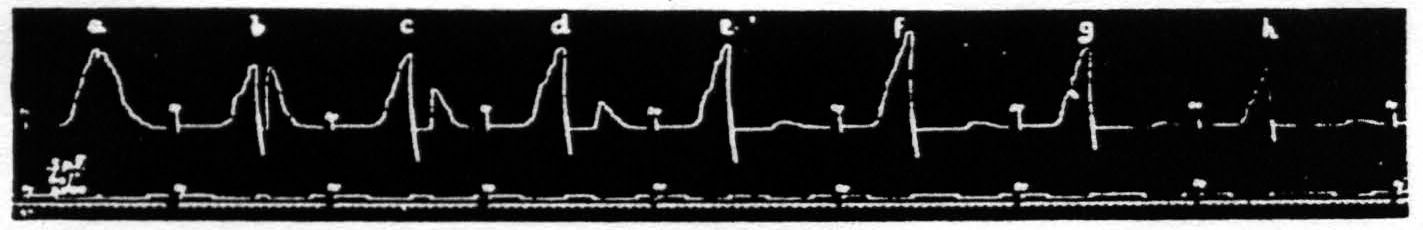
Figure 17. Oct. 16, 1936. Macaca mulatta. Dial-narcosis. This figure shows the dissociation of latency and amplitude in the response to constant test stimulation as a function of the interval after constant antecedent stimulation. Intermission between pairs = 2 min. Stimulus selected to avoid after-discharge.
Thus in the first half of Fig. 17 one has an admixture of facilitation and extinction, in the latter half pure extinction, i.e. by both criteria. In order to obtain such a record one has to use relatively long stimulation and one not giving cortical after-discharge, so that at the end of it many nerve cells have been discharged so often as to suffer the rise in threshold associated with positivity. In graph a, with an interval of only 0.2 of a second, the response to the second stimulation is practically a continuation of that to the first and declines during stimulation. Such a pair of stimulations may well be regarded as a single stimulation of double duration, the response to which exhibits extinction during stimulation. To account for this, one has to suppose that all the nerve cells which stimulation at this site, of this voltage and this wave form (pulse-frequency) can reach have become inexcitable to it. At such a time a response can still be elicited by stimulation of higher voltage or longer wave form (lower pulse-frequency), but not by one with higher pattern-frequency. Here it is important to point out a similarity to peripheral nerve, in that, so long as after-discharge is avoided, there exists for cortical stimulation a maximal stimulus in terms of change of any one parameter of stimulation except the voltage. The experiment just cited (Fig. 17) exhibits the justification of this statement in regard to the duration of stimulation. The exception concerning the voltage depends obviously upon the limited number of fibers in a peripheral nerve, whereas in the case of the cortex a higher voltage with consequent wider spread of current will always find more remote cells hitherto uninvolved.
3. Influence of parameters of stimulation
In a previous paper, dealing with pairs of equal stimulation, it was pointed out that, other conditions being equal, an increase in the total energy of stimulation by increase of any one of its parameters (duration, voltage, pattern-frequency or pulse-frequency) gave increase extinction(3) (p. 520). In that same paper it was further stated that with pairs of unequal stimulations the change in size of the motor response to the test stimulation is determined by each and every one of the parameters of both the antecedent and the test stimulation, as well, of course, as by their interval.
Though we cannot as yet show the relation of each of these nine variables to the temporo-spatial distribution of the three known factors for facilitation and extinction, we have now to present some findings in such experiments with pairs of unequal stimulations.
a. Pairs unequal in duration only. In the experiment of Fig. 18A four pairs of stimulations, the interval between members of each pair being 15 sec., were
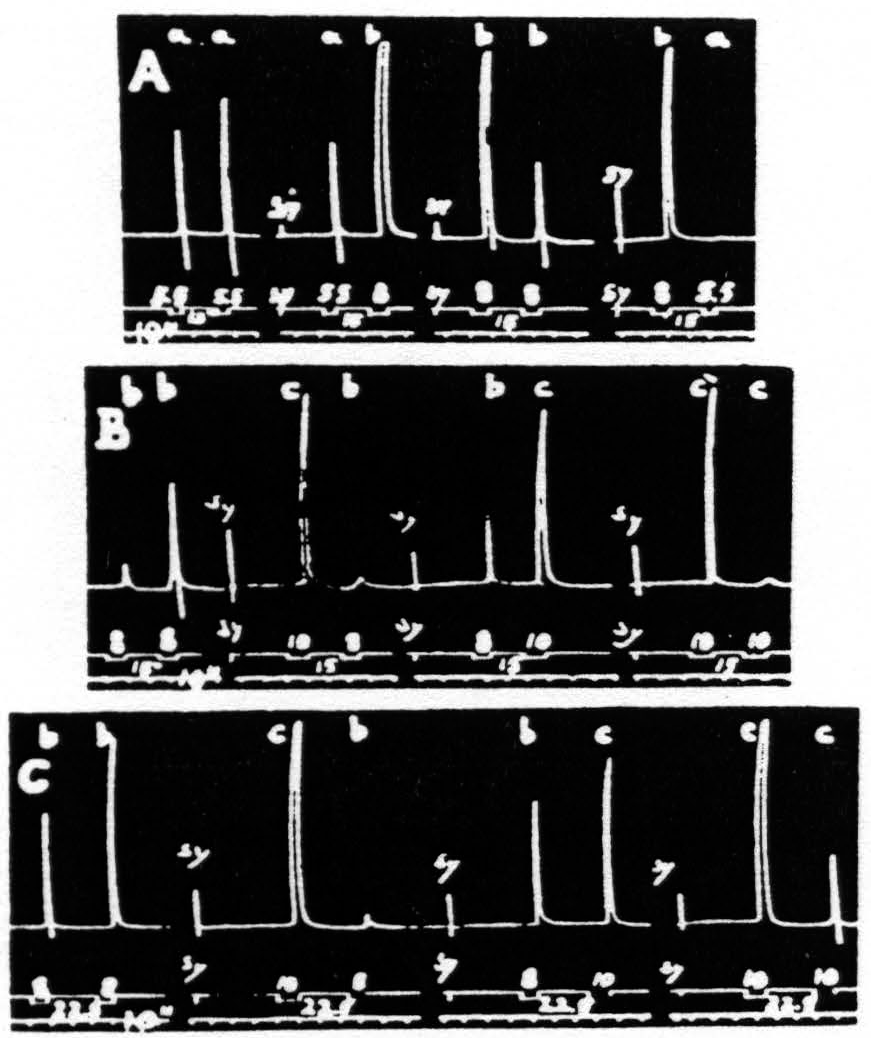
Figure 18. Dec. 2, 1938. Macaca mulatta. Dial-narcosis. Each record contains four pairs of stimulations, differing only in duration. Unchanged parameters of these stimulations: 40/″; .5µF; VD = 2150. The interval between members of each pair was in records A and B 15 sec., and in record C 22.5 sec. The durations in record A were 5.5 and 8 sec.; in records B and C, 8 and 10 sec. Note in record A that “a” facilitates “a” and “b”, “b” extinguishes “b” and “a”. In record B, “b” facilitates “ b”, facilitates “c” as to latency, extinguishes it as to amplitude, “c” extinguishes “b” and “c”. In record C it will be seen that “ b” facilitates “b”, but extinguishes “c”, while “c” extinguishes both “b” and “c”.
given. It will be seen that the shorter stimulation, “a”, always produced facilitation, the longer, “b”, always extinction; for the unaffected size of the response to “b” see responses 5 and 7. From this series it might seem as if the duration of the test stimulation is not significant; however, from Fig. 18B, where longer stimulations (8 and 10 sec. respectively) were used, it will be seen that the duration of the test stimulation must be considered. While the long stimulation, “c”, extinguishes both the response to itself, “c”, and to the short stimulation, “b”, and this stimulation, “b”, facilitates the response to itself, “b”, it produces a mixture of facilitation (shorter latency) and extinction (lesser amplitude) of the response to stimulation “c”. Records A and B were taken with the same interval (15 sec.) between members of each pair of stimulations.
In Fig. 18C, with the same type of stimulation as in Fig. 18B, but with the interval increased to 22.5 sec., it will be seen that now the effect of “b” on the response to “c” is extinction with regard to both latency and amplitude. The differences in results of Figs. 18B and 18C are in entire harmony with those of Fig. 17, showing the relation of the interval to the dissociation of latency and amplitude of the second response. The results of Fig. 18A and 18B, due to difference in duration of the stimulations (“a” and “b” versus “b” and “c”), are to be expected because the shortest of these, “a”, is such as to give predominantly facilitation, whereas “b”, being longer, produces somewhat more extinction, thus resulting in the admixture of facilitation and extinction (see Fig. 18B, pair “bc”), present also in Fig. 17.
In records like that of Fig. 18C, where one pair of stimulations produces one result and the other three the opposite, one is forced to conclude that the altered parameter of stimulation is significant in both antecedent and test stimulation. In the experiment of Fig. 18C it was the change in duration; in the subsequent Fig. 19, 20 and 21 the same will be shown for each of the other parameters.
b. Pairs unequal in pattern-frequency only. In experiments like the one depicted in Fig. 19 the only difference between the members of a pair of
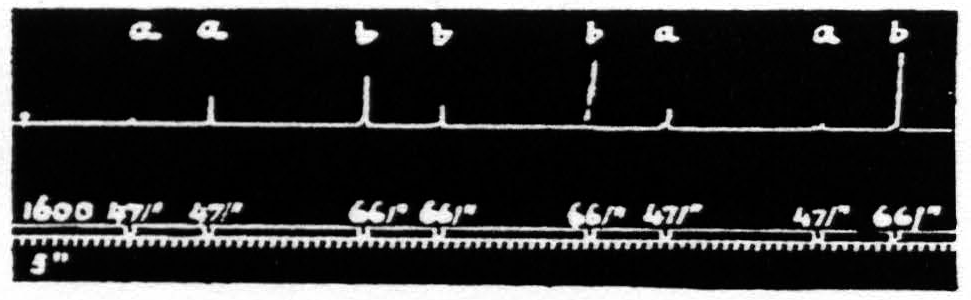
Figure 19. Feb. 12, 1935. Macaca mulatta. Dial-narcosis. This figure illustrates the effect of change in pattern-frequency alone. “a” facilitates ’’a” and “b”; “b” facilitates “a”, but extinguishes “b”.
stimulations was in the number of pulses per second (pattern-frequency ). By suitable selection of all parameters of the two stimulations and their interval it is possible to find such stimulations that those of lower pattern-frequency produce facilitation of response to themselves and to those of higher frequency, whereas those of higher pattern-frequency facilitate responses to those of lower frequency in spite of the fact that they produce extinction to themselves.
That in the pair “ba” the response to “a” is facilitated is clear by comparing this response with the first and seventh responses, which are unaffected by antecedent stimulation. This relation of these responses shows that the pattern-frequencies of both antecedent and test stimulation are significant.
c. Pairs unequal in pulse-frequency only. Figure 20 represents an experiment in which only the pulse-duration is altered. The stimulation with longer pulses, i.e. of lower pulse-frequency, always extinguishes, whereas stimulation with shorter pulses, i.e. of higher pulse-frequency, while it facilitates the response to itself, extinguishes the response to the stimulation of lower pulse-frequency.
That in the pair “ab” the response to “b” is extinguished is clear by
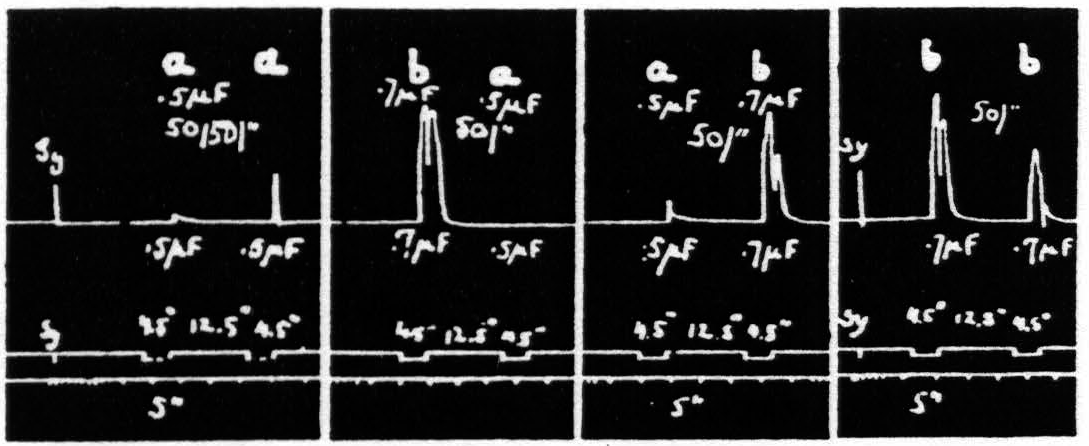
Figure 20. Feb. 21, 1939. Macaca mulatta. Dial-narcosis. This figure demonstrates Influence of change in pulse-frequency alone. “a” facilitates “a”, but extinguishes “b”: “6” extinguishes “a” and “b”.
comparing it with the third and seventh responses, which are unaffected by antecedent stimulation. Looking at this figure it is at once apparent that when all other parameters of stimulation are held constant, that having the lower pulse-frequency produces the larger response, i.e. that more motor-units have been excited. This presumably means that more cortical cells have been excited, rather than that a given group has been excited more often, for the pattern-frequency and duration of the stimulation were held constant. Since this experiment was made at constant voltage also, the voltage-gradient at any point in the cortex remains constant from stimulation to stimulation. Therefore, the increase in response with decrease of pulse-frequency is presumably due to an increase in the percentage of nerve cells excited within the area having a given voltage-gradient, rather than to any increase of that area. If this conception is correct, it must mean that the longer pulse-form excites a larger percentage of the cells by inclusion of cells with higher threshold. This is borne out by the comparatively slight effect of shorter pulses upon the responses to longer pulses, and the dominance of stimulation with longer pulses; for these can excite all the cells which the short pulses had excited, certainly if their threshold were lowered and even if it were somewhat raised. On the other hand it also implies that with longer pulses which can excite the vast majority of the nerve cells the decrease of excitability of a few of these will be sufficient to exhibit decrease of amplitude of response (extinction), whereas with shorter pulses there always remain enough nerve cells available for increase of amplitude of response (facilitation) even though many cells originally discharged have become inexcitable to these or longer pulses. Thus it should be possible to find a stimulation of shorter pulse-form which, though it produces facilitation of response to an equal test stimulation, produces extinction to test stimulation of longer pulse-form, as shown indeed in Fig. 20. Compare again the facilitation in record 1 and the extinction of response to “b” by “a” in record 3 using the response to “b” in record 2 or the first response to “b” in record 4 as standard.
d. Pairs unequal in voltage only. Figure 21 presents an experiment in which only the voltage is altered. The stimulation of lower voltage always facilitates, that of higher voltage, while it facilitates the response to stimulation of lower voltage, extinguishes the response to itself. This finding is in entire harmony with that presented in Fig. 16, in which stimulation at the higher voltage, which failed to produce facilitation as judged by increase of amplitude of response to itself, brought in a response to voltages previously subliminal.
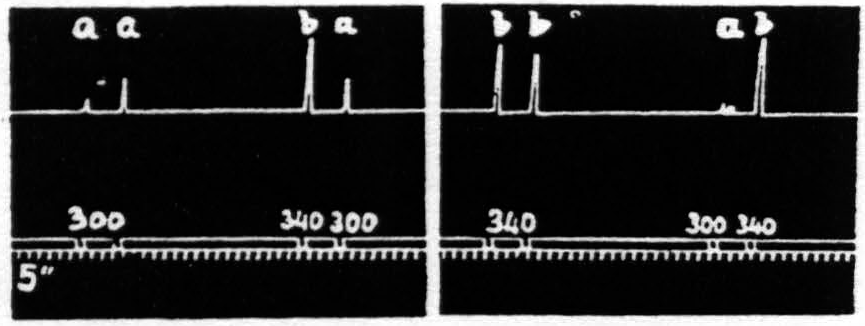
Figure 21. Feb. 14, 1935. Macaca mulatta. Dial-narcosis. This figure shows the effect of change in voltage alone. “a” facilitates “a” and “b”; “b” facilitates “a”, but extinguishes “b”.
That in the pair “ba” the response to “a” is facilitated is clear by comparing this response with the first and seventh responses, which are unaffected by antecedent stimulation.
In experiments wherein the voltage of stimulation is changed there is inherent a complexity not encountered in experiments with other inequalities of stimulation at a single focus. For not only is there with increase of voltage an increase in the percentage of nerve cells stimulated in the area reached by stimulation with lower voltage, but also an increase of the area affected (greater spread of current). To which of these an observed change of response is to be referred is, of course, uncertain, which precludes further analysis.
The changes in the population of nerve cells directly excited when voltage or pulse-form is changed, is a complexity absent when only pattern-frequency or duration is changed. Since with changes of one of the latter parameters alone the same population of nerve cells is excited—either more frequently by higher pattern-frequency or more often by longer duration—changes of threshold become of paramount importance and the results referable to the factors for facilitation and extinction.
4. Triple stimulation
At this point the findings with triple stimulation (see Fig. 3) must be considered in conjunction with parallel experiments on electrical activity and slow voltage drifts. The significant finding in such parallel experiments is that whereas the first stimulation initiates electrical after-discharge and negative voltage drift followed by positive voltage drift, the second stimulation elicits no electrical after-discharge but does produce a negative voltage drift which is consistently associated with decreased latency of the third (facilitated) response, irrespective of the amplitude of this response. This confirms one in the opinion that facilitation as expressed by decrease of latency of response is associated with decrease in threshold to repetitive stimulation, rather than with involvement of new neurons, excited trans-synaptically by after-discharge, which by summation is unquestionably a factor for increase of amplitude.
E. Predictions
1. Conditions for reversal of a cortical focus
All the facts and considerations enumerated above led to certain predictions concerning the apparent reversal of a cortical focus by antecedent stimulation of a related antagonistic focus. The predictions were that, irrespective of whether the stimulation of the focus to be reversed was predominantly facilitating or extinguishing at the interval under investigation, the reversal would occur provided 1. that the antecedent stimulation of the antagonistic focus produced strong primary facilitation at the interval in question; 2. that the primary facilitation of this antagonistic focus be such as to exhibit increase of amplitude or after-discharge and not merely decrease of latency.
This reversal of a cortical focus was designated above as an apparent reversal because the evidence presented led to the conclusion that the reversal does not depend upon any change at the focus reversed nor even upon a fall in threshold of the antagonistic focus, but upon persistence of activity involving lower levels of the CNS, e.g. the spinal cord. A reversal of response in which the cortex certainly plays a significant role has been observed with relatively prolonged stimulation of a single focus so as to extinguish it and facilitate a neighboring antagonistic focus. These predictions have been verified by the following results, all of which can be seen in one figure, namely Fig. 22. This figure presents six records, each consisting of four pairs of stimulations to two cortical foci, 3 mm. apart, one for extension (E) and one for flexion (F) at the elbow.
The antagonistic movements of the forearm at this joint were recorded with two tambours, the levers of which were attached with threads to the forearm just above the wrist. In the resting position these threads were just slack with the intention of obtaining independent records of the two motions.
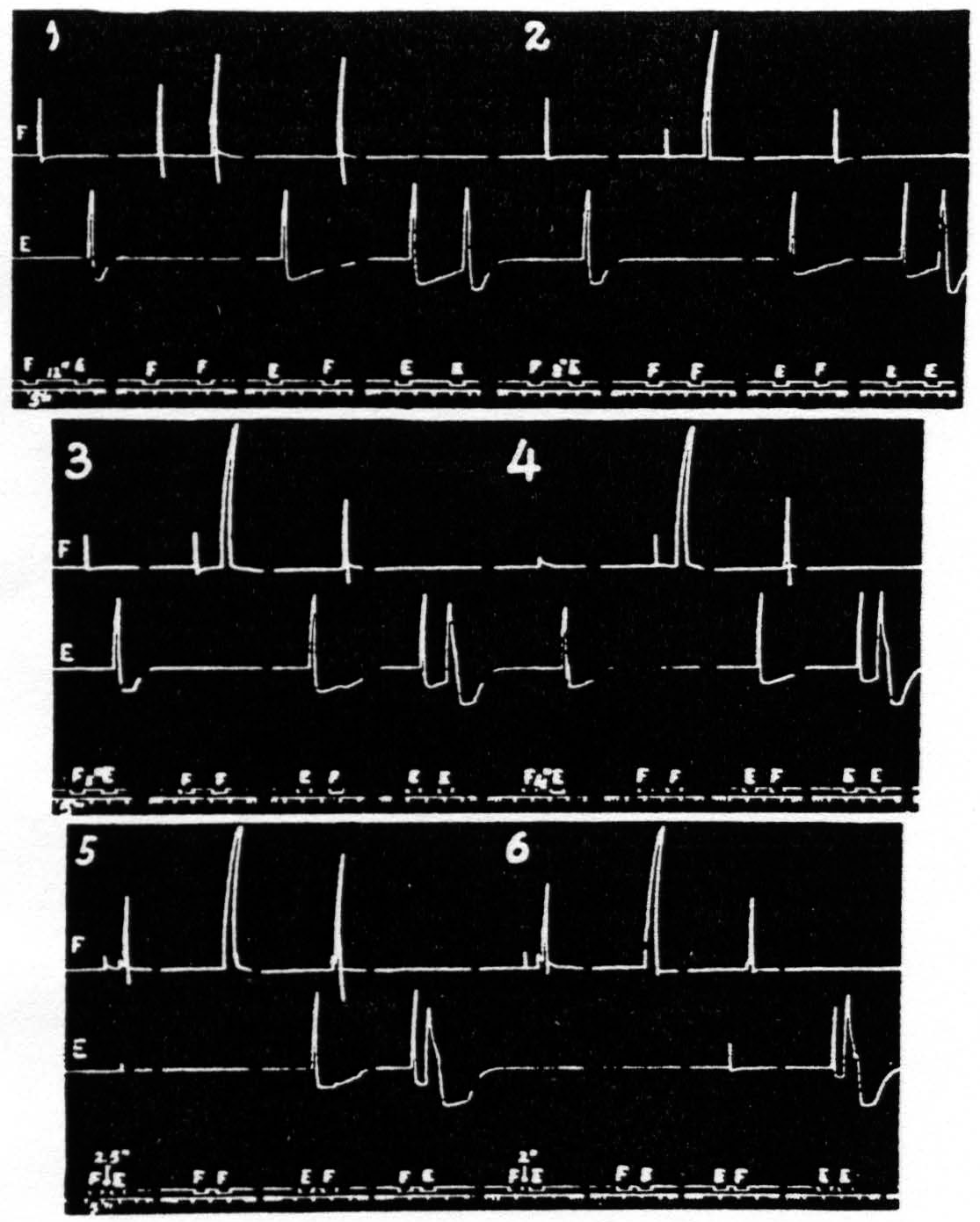
Figure 22. Jan. 26, 1937. Macaca mulatta. Dial-narcosis. This figure shows records of flexion and extension at elbow upon cortical stimulation of a flexor and an extensor focus respectively. In record 5 the appearance of a flexor response to E stimulation will be seen; for complete reversal see first graph of record 6. For further details see text. All parameters of stimulation constant throughout; only interval gradually shortened.
The excursions of the levers were recorded on the kymograph by means of two tambours. the upper recording the flexion, the lower the extension at the elbow. All stimulations were kept constant throughout the experiment and only the interval was decreased from record to record. The parameters of stimulation were chosen to produce primary facilitation at all intervals at the F focus, extinction with long and facilitation with very short intervals at the E focus.
Complete reversal can be seen in the first pair of responses (FE) of record 6, where stimulation of E 2 sec. after stimulation of F results in flexion only. This reversal is not obtained with antecedent extinguishing stimulation of the antagonistic focus (see pairs EF and EE of record 1), nor with weakly facilitating stimulation (see pairs FE and FF of records 1, 2, 3 and 4 but only with strongly facilitating stimulation (pairs FE and FF of records 5 and 6). Nor is facilitation as judged by decrease in latency alone sufficient to produce reversal (pairs EF and EE of records 2 through 6) even when, as in record 6. the latency is reduced to a minimum.
Thus, these findings fulfil the predictions that in order to obtain the apparent reversal of a cortical focus the stimulation of the antagonistic focus must be such as to produce primary facilitation, i.e. increased responsiveness to repetition of stimulation at that same focus, as judged by increase of amplitude (usually accompanied by motor after-discharge ) at the interval in question.
Scrutiny of these records brings out another interesting relation of the E and F responses. Reversal of a cortical focus necessarily includes diminution of the normal response to stimulation of the “reversed” focus, as shown by the gradual decline of the E responses in pairs FE in all records of Fig. 22 (as well as the appearance of the F response to the E stimulation ). This diminution cannot be considered as an extinction of this response, but must be interpreted as an inhibition in the extensor systems due to activity in the reciprocally related flexor systems, which finally in records 5 and 6 produce the F response to E stimulation. But that is not all. The E stimulation is predominantly extinguishing reaching its maximal effectiveness at an interval of 2.5 sec. The underlying decrease in activity of the extensor systems (extinction) should then manifest itself as an increased response to the F stimulation. That such is the case is seen in the increase in the F responses in the EF pairs of records 1 through 5. The decrease of this same response in record 6 at an interval of 2 sec. is caused by the appearance of some facilitation in the extensor systems at this short interval, seen in EE of this record.
One more point in regard to Fig. 22 should be noted. The stimulation to the F focus being facilitating, and the E stimulation not, there is always more excitation in the flexor than in the extensor systems. With decrease in interval and the eventual appearance of flexor response to the E stimulation this increased activity of the flexor system results in extinction in that system, as evidenced by the gradual decline, from record to record, in the size of the first response in each FF pair and their eventual disappearance in records 5 and 6. It is well to take this point into consideration in judging the amount of facilitation in these pairs in the successive records of Fig. 22.
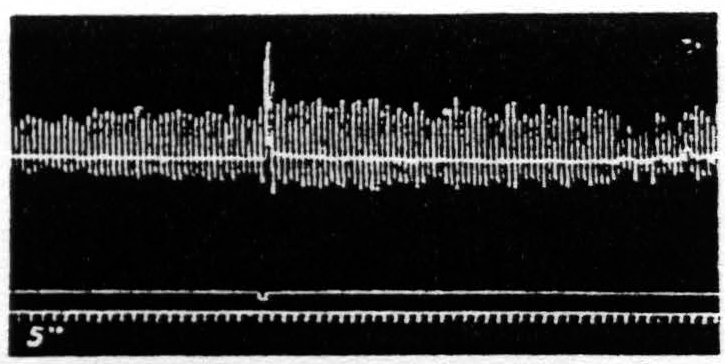
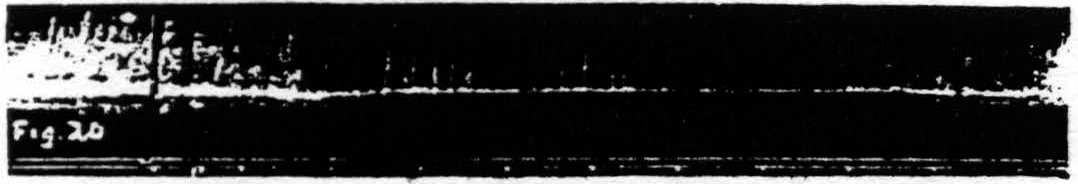
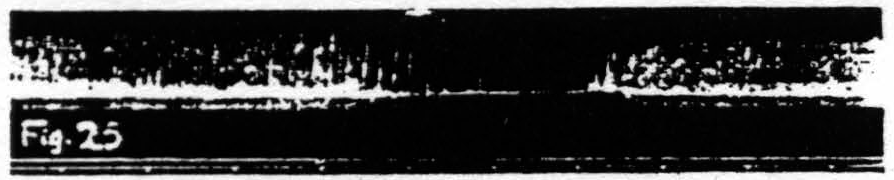

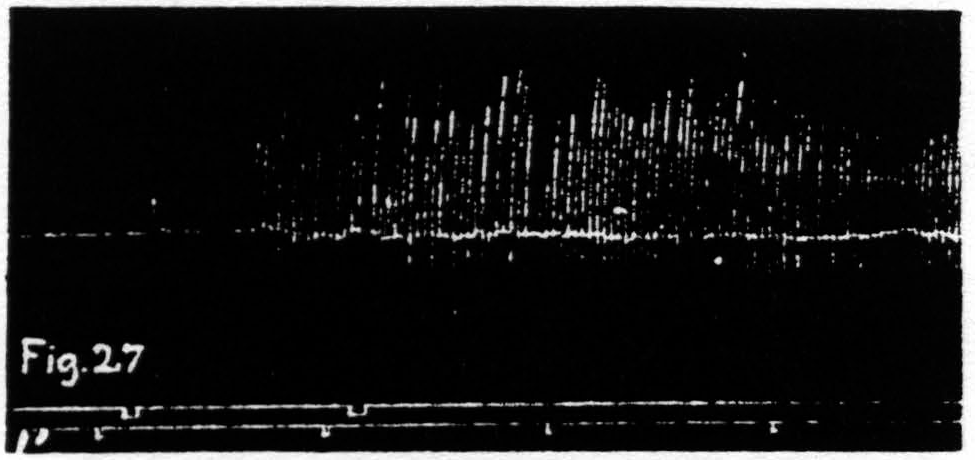
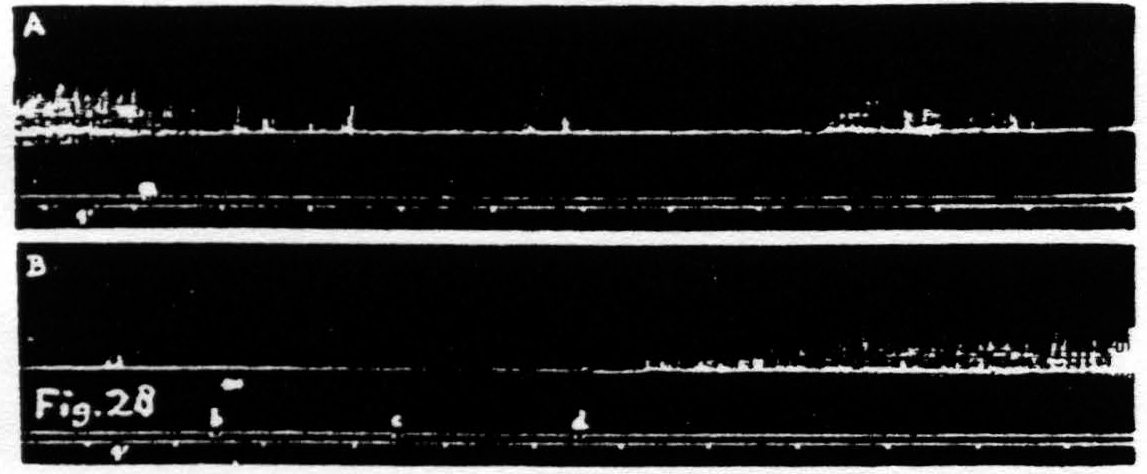

Figure 23. Feb. 18, 1937. Macaca mulatta. Dial-narcosis. Facilitation of knee-jerk by facilitating stimulation (0.3µF, 40/ʺ, 3 sec., VD = 5500) of a focus of L.4 for extension at the knee.
Figure 24. Nov. 17, 1938. Macaca mulatta. Dial-narcosis. Extinction of knee-jerk by extinguishing stimulation (0.6µF, 80/ʺ, 6 sec., VD = 1000) of a L.4 focus for extension at the knee.
Figure 25. Nov. 17, 1938. Same animal as of Fig. 22. Inhibition of knee-jerk by facilitating stimulation (0.3µF, 30/ʺ, 4 sec., VD =9000) of a L.4 focus for flexion at the knee.
Figure 26. Nov. 8, 1938. Macaca mulatta. Dial-narcosis. KJ elicited every 2 sec. Enhancement of KJ by extinguishing stimulation (1µF, 80/ʺ, 6 sec., VD =500) of an L.4 focus for flexion at the knee.
Figure 27. Nov. 17, 1938. Macaca mulatta. Dial-narcosis. KJ extinguished by extinction of an L.4 focus for extension at knee. KJ returns after facilitating stimulation of same focus.
Figure 28. Nov. 17, 1938. Same animal as of Fig. 25. In record A inhibition of KJ after facilitating stimulation (0.3µF, 30/ʺ, 4 sec., VD =7000) of a L.4 focus for flexion at knee. In record B return of KJ after repeated facilitating stimulation (1µF, 80/ʺ, 6 sec., VD =1000) of same focus.
Figure 29. Nov. 21, 1938. Macaca mulatta. Dial-narcosis. Disappearance of KJ after thermocoagulation at 80°C for 5 seconds of the area (3 mm.(15) )of L.4 from which extension at knee could be elicited. The KJ was absent for 15 hours and could then be brought back only by often repeated passive movements of leg at knee and even then its amplitude was small and irregular.
2. Changes in the knee-jerk
Consideration of the above findings with the observations of long lasting changes in activity induced in the spinal cord by cortical stimulation (see sub C.1.d. ), led to several predictions as to the effect of cortical stimulation on “spinal” reflexes. To test these predictions the knee-jerk (KJ) was elicited periodically(25) and changes in its amplitude induced by cortical stimulation recorded kymographically. The predictions were the following: 1. facilitating stimulation of a cortical focus for extension at the knee will produce a long lasting facilitation of the KJ; 2. extinguishing stimulation of the same focus will produce a prolonged diminution (extinction) of the KJ; 3. facilitating stimulation of an antagonistic cortical focus. yielding flexion at the knee. will result in a prolonged diminution (inhibition) of the KJ; 4. extinguishing stimulation of this same focus will enhance the KJ.
Inasmuch as this enhancement is due to factors for extinction in antagonistic systems we would avoid the term facilitation.
As corollaries to the foregoing it can be predicted that facilitating stimulation of an extensor focus and extinguishing stimulation of a flexor focus should bring back the KJ when it has been obliterated either by extinguishing stimulation of an extensor focus or facilitating stimulation of a flexor focus, and finally that passive movements at the knee will bring back by facilitation an extinguished or inhibited KJ.
Figure 23 shows the temporary facilitation of the KJ following facilitating stimulation of a focus for extension at the knee. Figure 24 shows the temporary extinction of the KJ following extinguishing stimulation of a focus for extension at the knee. Figure 25 shows the temporary inhibition of the KJ following facilitating stimulation of a focus for flexion at the knee. Figure 26 shows the temporary enhancement3 of the KJ following extinguishing stimulation of a focus for flexion at the knee. Figure 27 shows the return of the KJ by repeated facilitating stimulation of that focus for extension at the knee, extinguishing stimulation of which had extinguished the KJ. Figure 28 shows the return of the KJ by repeated extinguishing stimulation of that focus for flexion at the knee, facilitating stimulation of which had inhibited the KJ.
Each of the above predictions has, therefore, been verified. These findings in the KJ beautifully illustrate the importance of a proper balance of these reciprocally related flexor and extensor systems in the maintenance of the normal KJ. Obviously to these systems belong those flexor and extensor foci in the cortex, the stimulation of which, as shown above, profoundly changes the KJ. It became of interest, therefore, to investigate whether the destruction of one of these antagonistically, or reciprocally, related foci, leaving the other intact, would not so upset the balance of these systems as to alter the KJ demonstrably.
To answer this question the cortical area for extension at the knee of a monkey was mapped and found to be ca. 2 mm. in diameter. Extinguishing stimulation of the center of this area resulted in typical extinction of the KJ, which was reinitiated by passive flexions and extensions at the knee. After the KJ had returned to normal this extensor area was precisely thermocoagulated (80°C for 6 sec.), which after 2 min. resulted in an abrupt and almost complete obliteration of the KJ which became complete at the end of 4 min. Attempts to reinitiate it by passive movements at the knee resulted only in a few rapidly declining KJs (see Fig. 29). Extinguishing stimulation of the adjacent intact cortical flexor focus although resulting in prompt flexor responses at the knee failed to bring back the obliterated KJ. Fifteen hours after this thermocoagulation the KJ was still absent and when restored, after many attempts at facilitation by passive movements at the knee, was small and irregular. This experiment shows that in the monkey. even under narcosis, cortical impulses impinge upon the spinal cord and that cessation of impulses arising in a cortical focus for extension at the knee is sufficient to produce an imbalance between the extensor and flexor systems thus obliterating the KJ.
That it is a specific imbalance between the extensor and flexor systems rather than the elimination of non-specific cortico-spinal impulses, maintaining the general level of activity in the spinal cord, follows from the observation that thermocoagulation of the whole precentral leg area (L.4, L.4-S and L.6) results only in a very transient diminution of the KJ for circa 5 minutes, after which it returns to its original amplitude. The finding that a very circumscribed cortical lesion results in the loss of a reflex usually considered spinal and that an extensive destructive lesion of the cortex, including this area, does not result in this disorder is obviously of interest in regard to the problem of functional localization in the cortex.
Discussion
Before entering into the discussion it seems advisable to sum up the main points of the results presented. The first point is that it is now established that underlying facilitation and extinction are changes in the CNS induced by antecedent activity, not by mere passage of current. Second, that these changes differ in kind, in time and in space. Third, that they can operate only by affecting threshold and (or) activity and that in the living CNS a change in either of these inevitably induces a change in the other.
We have isolated for study the electrical activity, the slow potentials and the pH and examined these at the site of stimulation and at remote parts of the CNS, and correlated them with facilitation and extinction. This analysis has shown that increased activity, increased excitability associated with negative voltage drift and a probable alkalinity are factors for facilitation. that decreased activity, decreased excitability associated with positive voltage drift and acidity are factors for extinction. On the basis of these findings it was possible to explain some of the findings with pairs of unequal stimulations and to predict and verify conditions for “reversal” of a cortical focus and for changes in the knee-jerk induced by cortical stimulation.
The following points need more detailed consideration: (i) as stated in a previous paper (13, p. 523) it was Lorente de Nó who suggested that the diminution or absence of response to test stimulation might result from an absence of background-activity in reverberating circuits, because they have been forced to discharge simultaneously and, therefore, have become simultaneously refractory. The first part of this suggestion has been more than substantiated: for the rapid phase of an electrical after-discharge is always associated with facilitation, the diminution or absence of electrical activity following hyperactivity, whether due to direct stimulation or to an after-discharge propagated transsynaptically to the area under investigation, is always associated with extinction. Our technique has not been such as to allow verification of the second part of Lorente de Nó’s suggestion, except for this, that stimulation during the latter part of a prolonged electrical after-discharge regularly terminates it.
In this connection it is of interest to examine the typical form of an electrical after-discharge as exemplified in Fig. 10 in the record from area L.3. Following the long period during which this area has received impulses from the area stimulated (L.4) the active discharge of the area itself is characterized by a burst of rapid electrical fluctuations of remarkably constant form and interval; these end abruptly and are followed by much larger fluctuations of an entirely different form and lower frequency. This type of change may be repeated several times during a single after-discharge until the frequency has dropped to about 3 per sec. The end of the discharge may exhibit a gradual decline in voltage (as in Fig. 10), but often ends abruptly, in either case followed by diminution of electrical activity. This sudden drop in frequency associated with a change in wave form is what one would expect from Lorente de Nó’s work(26, 27, 28, 29) for short chains being discharged more frequently than long chains will sooner suffer a rise in threshold and cease to conduct, leaving only the longer circuits active.
This interpretation receives support from experiments on electrical after-discharge in monkeys before and after deep undercutting of the cortex. For, following such a lesion the picture of after-discharge is unchanged except for the absence of the slower, about 3 per sec. rhythms. This indicates that the circuits for the higher frequencies of after-discharge are purely cortical or cortico-cortical, whereas those for the low frequencies are relayed back to the cortex by subcortical or even lower structures.
This analysis explains why one finds extinction, not facilitation, during the slow, terminal phase of an after-discharge, for at this time too many cells have become inexcitable. As one would expect from this and as stated on p. 331 this latter part of an electrical after-discharge is associated with a positive voltage drift.
(ii). This brings us to the slow voltage changes that follow prolonged enforced activity. We not not wish to call these phenomena after-potentials; first, because, strictly speaking, they are not potentials in the sense of the physicist, and second, because the conditions for experimentation are not those for which an after-potential is defined, namely as alteration in the voltage of a current of injury (positive Nachschwankung of Ewald Hering(24)) in excised nerve. Nor do we wish to use Gasser’s designation(30) for somewhat similar phenomena in the spinal cord, which he calls intermediary potentials, not only because this again introduces the notion of potentials. but also because it refers the phenomena at hand to particular structures. namely internuncial neurones.
We are indebted to Dr. C. H. Prescott of the Bell Telephone Laboratories for calling to our attention that an electrical potential is not defined in the presence of a chemical reaction or where for any other reason there is a change in the carrier of the current, i.e. from an ion in solution to an electron in a wire or vice versa. What one observes under these conditions. i.e. in all ordinary electrophysiological experiments, are, strictly speaking. voltages, not potentials. For these reasons the designation “voltage drifts” seems to us the simplest, appropriate and noncommittal description. The term “drift” seems the more appropriate because these slow changes in voltage are presumably not the cause for the changes in threshold but merely one expression of those processes which underlie the accompanying changes in threshold.
Though these voltage drifts resemble in many points those observed in peripheral nerve and the spinal cord,(30) they are usually much larger and more prolonged. The negative drift in Fig. 13(15) for instance lasted about 20 sec., the ensuing positive drift between 5 and 10 minutes! Points of resemblance are: a. the sequence, i.e. negativity followed by positivity: b. the susceptibility to various conditions, such as temperature, poor local blood supply or poor general circulation, partial asphyxia. In our experiments on the cortex the depth of Dial-narcosis has been shown (Fig. 13) to be also of great importance. though we are not in a position to exclude entirely some element of lesser ventilation, since the animal breathed spontaneously. Finally these voltage drifts are augmented with increase of the number of neurons discharged and with the number of times they are discharged. This resemblance is not surprising if one considers the great number of nerve fibers in the cortex.
But irrespective of whether the processes underlying these voltage drifts are the same in cortex, cord and nerve, their association with the change in threshold to repetitive stimulation is always the same; negativity is associated with increased responsiveness, positivity with decreased responsiveness. It is of interest to point out that while the manner of picking up these voltage drifts on the cortex (without injury) is entirely different from that of picking up the after-potentials in an excised peripheral nerve (from longitudinal and cut surface), both are explicable in terms of the membrane-theory, for in both negativity is associated with a decrease and positivity with an increase of the “membrane-potential” in cells or fibers that have been discharged: the essential condition being that one electrode is at the site of this discharge, the other at an unaffected site, be it a remote focus in the cortex or the killed end of a peripheral nerve.
We have so far discussed the neural activity evidence by electrical activity at the time and place in question and the slow voltage drifts indicative of threshold changes then and there. Both of these changes are concerned only with neurons already involved. It was because it was impossible to frame a plausible explanation for the facilitation encountered in the experiments with unequal pairs of stimulations, and because of the persistence of diminished electrical activity and extinction beyond the period of positive voltage drift that we were forced to seek for a third variable, which would affect the threshold not only of neurons that had been discharged, but also the threshold of any neurons in their vicinity, whether previously excited or not. It was obvious that this variable must involve changes in the “milieu” of the neurons in question and was, therefore, presumably a change in ionic constitution. Obviously an investigation of the pH of the cortex was in order and, when made, the pH was found to be significant.
(iii). The specificity of the axonal terminals of the myriads of nerve cells in a given area of the cortex is so great that the consequences of a change in pH of a given area, carrying with it the alteration of activity of cells not involved in the response to a given stimulus, cannot be seen as yet in detail. Antagonistic cortical foci lie often so close together as to be affected contemporaneously by a local pH change. For this reason it is difficult to see how changes in pH could be responsible for the rapid contrasting phenomena encountered in reciprocal innervation, which imply changes of opposite sign in neighboring neurons. On the other hand the changes in threshold of a cell or cell-group associated with a slow voltage drift are discrete and limited to the cells discharged. In fact it is in terms of threshold changes of particular cells in the spinal cord that Gasser has offered an explanation of reciprocal inhibition. Thus it is possible to account for the inhibition encountered in reciprocal innervation in terms of a factor for extinction; it is obviously impossible to account for extinction in terms of inhibition, since extinction is a change in the system due to antecedent activity in that same system and, therefore, does not require a relation with an antagonistic system.
(iv). In conclusion we wish to mention briefly one more point. It is of interest to note that whereas the motor responses to constant intermittent electrical stimulation of a “motor” focus show typical “waves,”(23, 31) the spontaneous electrical activity of the cortex, without stimulation, does not show corresponding fluctuations. One is, therefore, driven to regard the occurrence of these waves as a consequence of the stimulation itself, i.e. as
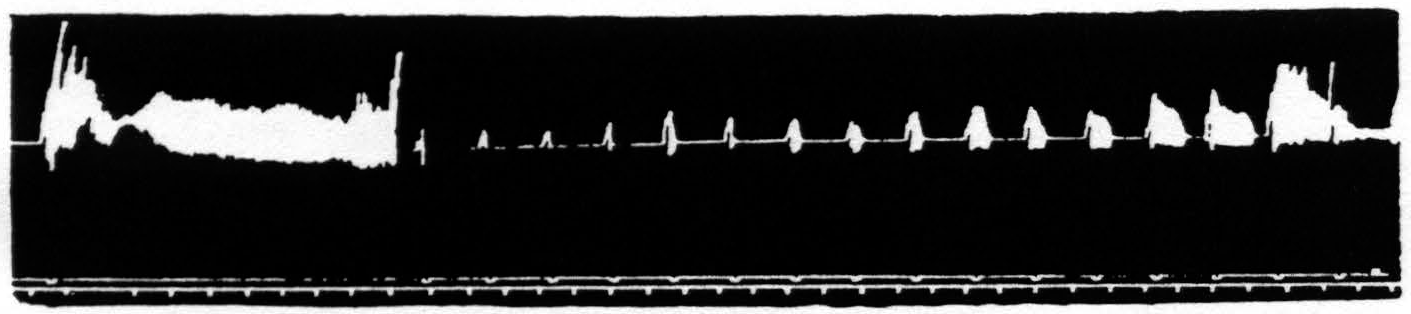
Figure 30. Feb. 26, 1936. Macaca mulatta. Dial-narcosis. Continuous subthreshold background stimulation at 5 per second of a “motor” focus for extension of wrist and intermittent repetitive stimulation (96 per sec.) at periods indicated by upper signal marker. Time 20 sec. The important thing to note is that the motor activity shown is not a motor after-discharge following the intermittent stimulations, but responses to the background stimulation facilitated by the intermittent stimulations. The second indicates rapid and slow changes in the responsiveness of the nervous systems involved. At the end of the record a new slow cycle begins; the whole record represents, therefore, a little more than one whole slow cycle of the order of 10 minutes’ duration.
being due to factors for facilitation and extinction. It is possible to verify this conclusion with the following experiment (see Fig. 30). One “motor” focus is stimulated from two independent sources, one of which delivers a background stimulation of 5 pulses per sec., which by themselves are just subthreshold. The other source delivers once every 34 sec. for a period of 3 sec., stimulation at 96 short pulses per sec. which when applied for the first time, and superimposed upon the background stimulation, results for 190 sec. in a series of responses to the latter stimulation. In Fig. 30 it will be seen that the first responses of this series are very large. then gradually diminish, then return to an intermediate size to end abruptly with a few large responses. During this period of responses to background stimulation the intermittent stimulation was omitted, so as not to interfere with the facilitated motor responses to the background stimulation. After the cessation of these responses the intermittent stimulation was reinstated and it will be seen 1. that each of these stimulations results in a temporary reappearance of motor responses to the background stimulation. 2. that these gradually become larger and more persistent. At the end of the record an entire cycle begins again. After all the foregoing the interpretation is not difficult. The first of the intermittent stimulations facilitates the response to the background stimulation in two ways: (i). by the relatively local changes of threshold and, ii). by the more widely spread after-discharge. The first of these runs a short course leading to extinction, producing a minimum of response at 40 sec. (positive drift at the site of stimulation), whereas the second runs a much longer course (typical of electrical after-discharge in cortex and cord) to end abruptly in extinction (extinction due to involvement of remote cortical foci and spinal structures). The dissipation of this extinction is clearly exhibited in the latter two-thirds of the record. It is important to note that the prolonged facilitation which parallels an electrical after-discharge cannot be repeated for many minutes, whereas the short-lived facilitation associated with negative voltage drift is repeatable within about half a minute after the end of the facilitated responses associated with electrical after-discharge. This again confirms the finding mentioned before (p. 343).
Figure 30 shows that regular intermittent repetitive stimulation of the cortex sets up slow rhythmical changes in its responsiveness and indicates that underlying the “waves” of cortical excitability are the more persistent factors for facilitation and extinction in parts of the CNS distant from the site of stimulation. These are, for facilitation, the electrical after-discharge and its associated slow negative voltage drift (and probably alkalinity) and, for extinction. the diminution of electrical activity, its associated slow positive voltage drift and certainly acidity.
Conclusions
Facilitation and extinction are alterations induced in the response to test stimulation by antecedent stimulation. Underlying facilitation and extinction are changes in the activity and threshold of the parts of the CNS involved in the response to test stimulation. These changes, initiated by antecedent excitation, differ in kind and vary in intensity from time to time and from place to place.
For facilitation the factors are: (i). hyperactivity yielding increased summation; (ii). negative voltage drift associated with decrease of threshold in neurons previously involved; (iii). probably increase of pH (alkaline shift) decreasing the threshold of all neural structures in the region involved. For extinction the factors are: (i). hypoactivity resulting in less summation: (ii). positive voltage drift associated with increase of threshold in neurons previously involved; (iii). decrease of pH (acid shift), increasing the threshold of all neural structures in the region involved.
The effects of choice of the parameters of antecedent and test stimulation and of the interval between them upon latency, threshold and amplitude of response are explicable in terms of the modes of action of these factors.
The findings presented in this paper offer an approach to the problem of reciprocal innervation, for they compel now verified prediction of (i). the conditions for apparent reversal of a cortical focus, and (ii). the changes in the knee-jerk following facilitating or extinguishing stimulation of a cortical focus for extension or flexion at the knee. Inhibition in reciprocal innervation is explicable in terms of the more discrete factors for extinction.
The “waves” of cortical excitability, previously described, are referable to the more remote and prolonged factors for facilitation and extinction.
Footnotes
References
Bubnoff, N., and Heidenhain, R. Ueber Erregungs- und Hemmungsvorgänge innerhalb der motorischen Hirncentren. Pflug. Arch. ges. Physiol., 1881, 26: 137-200.
Dusser de Barenne, J. G., and McCulloch, W. S. An “extinction” phenomenon on stimulation of the cerebral cortex. Proc. Soc. exp., Biol N.Y., 1934, 32: 524-527
Dusser de Barenne, J. G., and McCulloch, W. S. Local stimulatory inactivation within the cerebral cortex, the factor for extinction. Amer. J. Phvsiol., 1937, 118: 510-524.
Schmitt. O. H. A., and Schmitt, F. O. A universal precision stimulator. Science. 1932, 76: 328-330.
Burr, H. S., Lane, C. T., and Nims, L. F. A vacuum tube microvoltmeter for the measurement of bioelectric phenomena. Yale J. Biol. Med., 1936, 9: 65-76.
Dusser de Barenne. J. G., McCulloch. W. S.. and Nims, L. F. Changes of hydrogen ion concentration of the cerebral cortex. Proc. Soc. exp. Biol. N.Y., 1937, 36: 462-464.
Dusser de Barenne, J. G., McCulloch, W. S., and Nims. L. F. Functional activity and pH of the cerebral cortex. J. cell. comp. Physiol., 1937, 10: 277-289.
McCulloch, W. S., and Wendt, G. R. Photokymographic method with continuous cathode ray oscillograms. Science, 1936, 83: 354-355.
Dusser de Barenne, J. G., and McCulloch, W. S. Functional organization in the sensory cortex of the monkey (Macaca mulatta). J. Neurophysiol., 1938, 1: 70-85.
Lane, C. T., McCulloch, W. S., Prescott, C. H., and Dusser de Barenne. J. G. A new method for the investigation of the electrical properties of living tissues. Amer. J. Physiol., 1935, 113: 85.
Dusser de Barenne, J. G. Simultaneous facilitation and extinction of motor response to stimulation of a single cortical focus. Amer. J. Physiol., 1936, 116: 39-40.
Dusser de Barenne, J. G. Simultane Bahnung und Auslöschung in der “motorischen” Hirnrinde. Conf. neurol., 1938, 1: 1-4.
Mcculloch. W. S., and Dusser De Barenne, J. G. Action potentials of the cerebral cortex and spinal cord before and after cortical stimulation. Amer. J. Physiol., 1936, 116: 99.
Dusser de Barenne, J. G., and Mcculloch, W. S. Kritisches und Experimentelles zur Deutung der Potentialschwanktungen des ElektrocorticogTamms. Z. ges. Neurol. Psychiat., 1938, 162: 815-824.
Bartley, S. H., and Bishop, G. H. The cortical response to stimulation of the optic nerve in the rabbit. Amer. J. Physiol., 1933, 103: 159-172.
Bishop, G. H. Cyclic changes in excitability of the optic pathway of the rabbit. Amer. J. Physiol., 1933, 103: 213-224.
Bishop, G. H. La théorie des circuits locaux permet-elle de prévoir la forme du potentiel d’action? Arch. int. Physiol., 1937, 45: 273-297.
Bishop, G. H., and O’Leary, J. Components of the electrical response of the optic cortex of the rabbit. Amer. J. Physiol., 1936, 117: 292-308.
Marshall, W. H., Woolsey, C. N., and Bard, P. Cortical representation of tactile sensibility as indicated by cortical potentials. Science, 1937, *85 *: 388-390.
Marshall. W. H., Woolsey, C. N., and Bard, P. in MacLeod, Physiology in modern medicine, 8th ed. St. Louis. C. V. Mosby and Co., 1938 (see 168-173. 194).
Adrian, E. D. The spread of activity in the cerebral cortex. J. Physiol., 1936, 88: 127-161.
Dusser de Barenne, J G., Marshall, C. S., McCulloch. W. S., and Nims, L. F. Observations of the pH of the arterial blood, the pH and the electrical activity of the cerebral cortex. Amer. J. Physiol., 1938, 124: 631-636.
Brody, B. S., and Dusser De Barenne, J. G. Effect of hyperventilation on the excitability of the motor cortex in cats. Arch. Neurol. Psychiat., Chicago, 1932, 28: 571-585.
Hering, E. Beiträge zur allgemeinen Nerven- und Muskel-physiologie. XV. Mittheilung. Über positive Nachschwankung des Nervenstromes nach elektrischer Reizung. S.B. Akad. Wiss. Wien., 1884, 87: 137-158.
Dusser de Barenne, J. G., and Ward, A. A., JR. Reflex inhibition of the knee-jerk from intestinal organs. Amer. J. Physiol., 1937, 120: 340-344.
Lorente de Nó, R. The synaptic delay of the motoneurones. Amer. J. Phvsiol.. 1935, 111: 272-282.
Lorente de Nó, R. The refractory period of the motoneurones. Amer. J. Phvsiol., 1935, 111: 283-288.
Lorente de Nó, R. Facilitation of motoneurones. Amer. J. Phvsiol., 1935, 113: 505-523.
Lorente de Nó, R. Analysis of the activity of the chains of internuncial neurons. J. Neurophysiol., 1938, 1: 207-244.
Gasser, H S., and Graham, H. T. Potentials produced in the spinal cord by stimulation of dorsal roots. Amer. J. Physiol., 1933, 103: 303-320.
Hovland, C. I., and Dusser De Barenne. J. G. Periodic fluctuations in motor response to uniform electrical Stimulation of the cerebral cortex in monkeys. Amer. J. Physiol., 1936, 116: 79.
Dusser de Barenne, J. G., and McCulloch, W. S. Extinction: local stimulatory inactivation within the motor cortex. Amer. J. Physiol., 1935, 113: 97.
Gasser, H. S. Changes in nerve-potentials produced by rapidly repeated stimuli and their relation to the responsiveness of nerve to stimulation. Amer. J. Physiol., 1935, 111: 35-50.
Gasser, H. S. The control of excitation in the nervous svstem. Han. Led., 1937, 32:169-193.
McCulloch, W. S. On the nature and distribution of factors for facilitation and extinction in the central nervous system. Amer. J. Physiol.. 1937, 119: 363-364.
McCulloch. W. S., and Dusser De Barenne, J. G. The knee-jerk following facilitating or extinguishing stimulation of related cortical foci. Amer. J. Physiol., 1939 (in press).
Epi-search analysis
Word cloud: Activity, After-discharge, Amplitude, Antecedent, Area, Associated, Cells, Changes, Cns, Cortex, Cortical, Decrease, Drift, Electrical, Electrodes, Excitation, Experiments, Extinction, Extinguishing, Facilitation, Factors, Fig, Figure, Foci, Focus, Following, Increase, Interval, Knee, Latency, Macaca, Motor, Mulatta, Negative, Nerve, Pairs, Period, Physiology, Positive, Produce, Record, Response, Results, Shows, Slow, Stimulation, Systems, Test, Threshold, Voltage
Topics: Stimulation, Response, Facilitation, Antecedent, Extinction, Excitation, Interval, Factors
Keywords:
Stimulation, Response, Limb, Cortex, Phenomenon, CNS, Physiology, Excitation, Extinction, Facilitation (final)
Stimulation, Voltage, Response, B, Stimulations, CNS, Limb, Cortex, After-discharge, Nims (full text 1)
Stimulation, Electrodes, Cortex, CNS, Facilitation, Activity, Response, Cathode, Extinction, Motor (full text2)
Citations:
Related Articles:
Related books:
Keywords from the citations and related material: Stimulation, Brain, Cortex, Plasticity, Activity, Neurons, Motor, Response, Hippocampus, Facilitation